DME, Steroids and Glaucoma
Addressing concerns about steroid-induced intraocular pressure increases and the risk of glaucoma when managing diabetic macular edema
At a Glance
- Intravitreal corticosteroids are associated with risks, including IOP elevation which occurs in approximately 30–40 percent of treated patients
- Elevated IOP alone does not constitute glaucoma, and when secondary to an intravitreal steroid injection, is usually readily manageable with observation or topical ophthalmic drops in the large majority of cases
- For appropriate DME patients, intravitreal corticosteroids can represent a valuable alternative treatment option, with the possibility of a decreased treatment burden.
In my practice, I consider using intravitreal corticosteroids for managing diabetic macular edema (DME) in two categories of patients. First, among patients who respond adequately to anti-VEGF therapy, but the durability of current generation anti-VEGF monotherapies is insufficient for their lifestyle or preference. In this context, utilization of a steroid implant can often achieve a decreased treatment burden. Second, among patients who demonstrate an incomplete response to adequate anti-VEGF dosing.
There are legitimate reasons to pause before initiating corticosteroid therapy for DME. First, they are well recognized to increase the risk of cataract acceleration. In my view, even a single intravitreal steroid injection permanently changes the trajectory of cataract progression in that eye. Second, they have the potential to increase intraocular pressure (IOP); but there can be misconceptions about the correlation between IOP and glaucoma.
Elevated IOP alone does not constitute glaucoma. This simple distinction is often confused on the podium and in the published literature. While more data is needed related to the scenario of elevated IOP following pharmaceutical interventional, multiple studies have considered the complex relationship of absolute IOP and glaucoma. For example, the Baltimore Eye Survey involving 5,308 individuals (ages 40 years and older who underwent detailed ocular exams including perimetry), reported that even with an IOP of 30 mmHg, the majority of patients (95 percent) did not have glaucoma (1). Additionally, the Ocular Hypertension Treatment Study (OHTS) reported that with an untreated IOP of 24 to 32 mmHg, 9.5 percent of participants developed primary open-angle glaucoma (POAG) after 5 years of follow-up; this rate was reduced in OHTS to 4.4 percent among participants randomized to use of a topical ocular hypotensive medication regimen (2) (Figure 1).
Recognition that elevated IOP is not a surrogate for a diagnosis of glaucoma was highlighted by the removal of IOP as part of the definition of POAG in the American Academy of Ophthalmology (AAO) 2015 Preferred Practice Pattern® Guidelines for POAG (3). The current definition reads in part:
“POAG is defined as a chronic, progressive optic neuropathy in adults in which there is a characteristic acquired atrophy of the optic nerve and loss of retinal ganglion cells and their axons.”
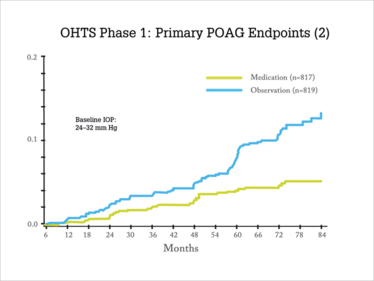
Figure 1. OHTS was a randomized trial conducted at 22 clinical centers. A total of 1,636 participants 40 to 80 years of age, with no evidence of glaucomatous damage and an lOP between 24–32 mm Hg in one eye and 21–32 mm Hg in the other eye were randomized to either observation (n=819) or treatment with commercially available topical ocular hypotensive medication (n=817). The goal in the medication group was to reduce the lOP by 20 percent or more and to reach an lOP of 24 mm Hg or less. The primary outcome was the development of primary open-angle glaucoma (POAG) in one or both eyes (2). Log rank P value < .0001, hazard ratio 0.40, 95% confidence interval (0.27, 0.59). Cumulative proportion POAG at 60 months, 9.5 percent in observation group and 4.4 percent in medication group. Adapted from (1)(2)(11).
Elevating the discussion of steroid-induced IOP
While elevated IOP is not synonymous with glaucoma, that does not negate the real clinical concern that steroid treatments can and often do increase IOP in treated patients. Specifically, approximately 30-40 percent of patients treated with intravitreal steroids will experience a clinically relevant elevation of IOP. In consideration of how to manage such an elevated IOP clinically, it may help to consider what we know about its cause and its course in reported prospective clinical trials.
The pathophysiology of IOP elevation following intravitreal steroid treatment is incompletely understood. It is hypothesized that such elevation may be related to increased outflow resistance (4). Specifically, increased IOP may be facilitated by modulation of glucocorticoid receptors within trabecular meshwork cells, theoretically altering the rate of protein synthesis and inhibiting degradation of the extracellular matrix (ECM) (5). However, other studies have reported the opposite (6).
Some of the clinical factors to consider when determining if steroid treatment may be appropriate include historical points such as a personal or immediate family history of POAG, or a history of steroid-induced IOP elevation. Examination findings to consider include higher baseline IOP, high myopia, evidence of glaucomatous nerve damage even in the absence of a definitive diagnosis of glaucoma, and evidence of angle recession. In any of these situations, I am typically more hesitant to consider administering intravitreal steroids.
When treating patients with intravitreal steroids, data from the prospective MEAD and FAME trials are good sources of information to gauge risk and guide management of IOP elevation. In the MEAD trial, the pooled results from two randomized, sham-controlled, 3-year studies of 347 0.7 mg dexamethasone- and 350 sham-treated patients found that 27.7 percent of patients given dexamethasone had an IOP increase >10 mmHg from baseline; 41.5 percent were prescribed IOP-lowering drops compared to 9.1 percent of control patients. The study also found that two patients (0.6 percent) in the 0.7 mg group underwent surgical intervention for elevated IOP, one attributed to steroid-induced IOP elevation and one to neovascularization of the anterior segment (7, 8). Additionally, the collective results of the 36-month FAME trials, which consisted of two randomized, sham-controlled, phase III studies reported that 38.4 percent of 0.2 µg/day fluocinolone acetonide treated patients were prescribed IOP-lowering drops compared to 14.1 percent of control patients. The study also found that while 4.8 percent of 0.2 µg/day fluocinolone acetonide treated patients underwent incisional surgery for elevated IOP, prior ocular steroid treatment correlated strongly with this risk; specifically, no 0.2 µg/day fluocinolone acetonide-treated patients who received prior ocular steroids required IOP-lowering surgery (9). This finding contributed to the FDA-approved package insert for fluocinolone for DME which requires that patients be “previously treated with a course of corticosteroids” and not have “a clinically significant rise in IOP” (10).
Focusing on management
In my own practice, patients treated with intravitreal corticosteroids are typically monitored every one to two months initially and then at longer intervals, generally not exceeding 3 months, after achieving disease stability. When a patient presents with elevated IOP following intravitreal steroid delivery, how I manage them depends on the degree of IOP elevation and their individual baseline risk profile (11). When possible, since an individual’s IOP can vary, correctly assessing levels and potential treatments are ideally based on several IOP measurements rather than a single assessment. In most cases, I will obtain a retinal nerve fiber layer (RNFL) OCT scan if not already obtained; this allows me to follow their RNFL longitudinally for evidence of thinning.
In the setting of mild IOP elevation, I will often simply observe the patient without intervention and in many cases the IOP will return to baseline as the effect of the steroid diminishes. If IOP rises to above 30 mmHg, regardless of baseline risk status, I often treat with a topical IOP-lowering medication. If IOP has risen more than 10 mmHg but is not above 30 mmHg, I will follow these eyes closely often without treatment. When initiating topical therapy, I typically will use an aqueous suppressant. If intravitreal steroid therapy is continued, I will continue the topical IOP-lowering medication; if intravitreal steroid therapy is discontinued, I will often discontinue the topical IOP-lowering medication once the IOP has normalized. In most cases, steroid-induced elevated IOP can be treated with topical IOP-lowering medications. In cases where alternative or additional treatment is required, laser trabeculoplasty and surgery that could involve either a trabeculectomy or tube shunt are options to consider; I often co-manage these patients with either referring ophthalmologists or referring optometrists comfortable with managing IOP.
Summary
With an appreciation of the multiple pathophysiologic pathways involved in DME, corticosteroids can play a role in its management in certain patient populations. Based on current evidence and my experience with patient responses to intravitreal corticosteroids, while IOP elevation is a common clinical reality in treated patients, these elevations are typically readily managed with either observation or topical IOP-lowering medications. Isolated IOP elevation is not equivalent to a diagnosis of glaucoma. The potential benefits of corticosteroids need to be weighed against the risks of cataract acceleration and IOP elevation for each individual patient.
Charles C. Wykoff is Director of Research at Retina Consultants of Houston; Deputy Chair for Ophthalmology at Blanton Eye Institute; and Associate Professor of Ophthalmology at Weill Cornell Medical College, Houston Methodist Hospital, Houston, Texas, USA.
Wykoff reports the following disclosures: Consultant for Alimera Sciences, Allergan, Bayer, Clearside Biomedical, D.O.R.C. International, Genentech, Novartis, Regeneron, Roche; Speaker for Allergan, Regeneron; and Research Support from Aerpio, Alcon, Allergan, Apellis, Clearside Biomedical, Genentech, Heidelberg, NEI, Novartis, Ophthotech, Regeneron, Roche.
- A Sommer et al, “Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans”, Arch Ophthalmol, 109, 1090–1095 (1991). PMID: 1867550.
- MA Kass et al., “The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma”, Arch Ophthalmol, 120, 701–713 (2002). PMID: 12049574.
- AAO PPP Glaucoma Panel. “Primary open-angle glaucoma PPP - 2015”. Available at: bit.ly/2nDB9Eg. Accessed: August 14, 2018.
- TF Freddo and H Gong, “Etiology of IOP elevation in primary open angle glaucoma”, Optometric Glaucoma Society E J, 4 (2009). PMID: 22957318.
- X Zhang, et al., “Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-beta”, Invest Ophthalmol Vis Sci, 46, 4607–4616 (2005). PMID: 16303956.
- Z Jing-ying Z, et al., “Reversible changes in aqueous outflow facility, hydrodynamics, and morphology following acute intraocular pressure variation in bovine eyes”, Chinese Med J, 126, 1451–1457 (2013). PMID:23595376.
- RK Maturi, et al., “OZURDEX® MEAD Study Group: Intraocular pressure in patients with diabetic macular edema treated with dexamethasone intravitreal implant in the 3-year MEAD study”, Retina,23 1143–1152 (2016). PMID: 26871523.
- DS Boyer,et al., “OZURDEX® MEAD Study Group: Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema”, Ophthalmology, 121, 1904–1914 (2014). PMID: 24907062.
- RK Parrish, et al., “FAME Study Group: Characterization of intraocular pressure increases and management strategies following treatment with fluocinolone acetonide intravitreal implants in the FAME trials”, Ophthalmic Surg LasersImaging Retina, 47, 426–435 (2016). PMID: 27183546.
- ILUVIEN prescribing information.
- BE Prum, et al., “Primary open-angle glaucoma suspect: Preferred Practice Pattern® Guidelines”, Ophthalmology, 123, P112–P151 (2016). PMID: 26581560.
Charles C. Wykoff is Director of Research at Retina Consultants of Houston; Deputy Chair for Ophthalmology at Blanton Eye Institute; and Associate Professor of Ophthalmology, Weill Cornell Medical College, Houston Methodist Hospital, Houston Texas.













