
Shock Treatment
Can electrostimulation of the ciliary muscle delay the loss of accommodation experienced in early presbyopia?
At A Glance
- There are a number of options for the treatment of presbyopia, which range from spectacle/ contact lens use, to multifocal intraocular lens or corneal inlay implantation
- Electrostimulation of the ciliary body might be the latest (nonsurgical) addition to that list
- An initial trial found that the procedure was not painful, had no apparent side effects, and appeared to positively impact presbyopia
- It turns out that demonstrating improvements in accommodation is technically challenging – but was achieved by combining anatomical, subjective and objective data
Old age brings with it an ocular inevitability: presbyopia. Everyone becomes presbyopic to some degree as they age. Today, it can be dealt with non-surgically with spectacle or contact lens use, or surgically with procedures like clear lens exchange, corneal inlay implantation, and a number of laser refractive approaches. But another strategy for correcting presbyopia is under development – electrostimulation therapy to restore the accommodation of the lens and the ciliary body.
Swimming against the current?
Electrostimulation of the eye isn’t a new concept – previous studies have explored its applications in glaucoma, retinal dystrophy, AMD, and progressive myopia, and it’s garnered some positive results with few reported side effects (1)(2)(3). However, this isn’t a popular area of research – a search using the terms “electrostimulation” AND “eye” in PubMed produces only 25 results.
Presbyopia is caused by two factors – as people age, their ciliary muscles progressively weaken, and their crystalline lenses start to lose elasticity. Resorting to near-vision glasses only weakens the ciliary muscle further (as it starts to become “lazy”), meaning that people become increasingly dependent on those spectacles with time. But what if we could give the ciliary muscle a workout? We propose that electrostimulation can be used to restore the loss of accommodation experienced by patients with early presbyopia – and we set out to study the efficacy of micro-electrostimulation of the ciliary body as a noninvasive presbyopia treatment.
We enrolled people aged between 40 and 50 years, who had early presbyopia no greater than +1.50 D, and were either emmetropes or low hyperopes. We excluded pseudophakes and those with any ocular pathologies or neuropathies, those on medications that could influence the accommodative response, and people with demyelinating or vascular diseases that might affect the ocular influx to the ciliary body. People with cardiac pacemakers or who were affected by epilepsy were also excluded.
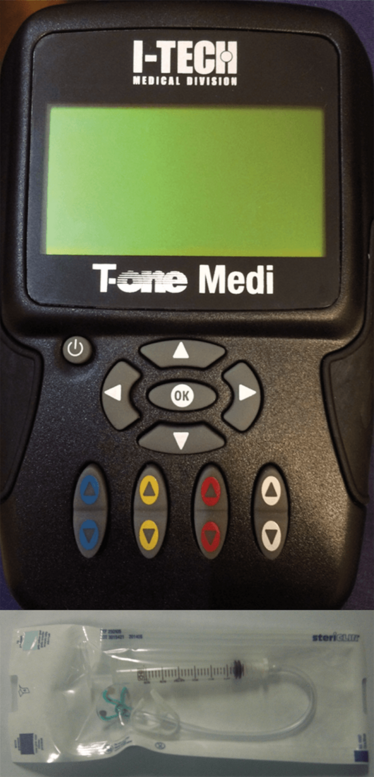
Figure 1. Components of the electrostimulator kit (CE 0051) including a contact lens, syringe, and current generator.
A ciliary body workout
The medical device we use to carry out this procedure is CE marked, and comprises a contact lens, a syringe (which can be used for suction, but which is optional), a cable, and a generator that supplies a square wave biphasic compensated micro-continuous electrical current (Figure 1). The current induces passive exercise of the ciliary body by generating a rhythmic, low-voltage, contraction and relaxation of the ciliary muscle. During the treatment there is also a rhythmic contraction of the pupillary muscles, which alternate between miosis and mydriasis – the “workout.”

Figure 2. The contact lens 20 mm-diameter rigid scleral lens is connected to four 3 mm electrodes, which are in turn directly connected to the electrostimulating device.
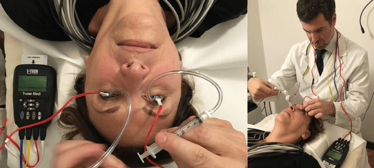
Figure 3. Bilateral electrostimulation using the device.
The contact lens we use is a 20 mm-diameter rigid polycarbonate scleral lens (Figure 2). The internal side is placed in contact with the bulbar conjunctiva – but makes no contact with the cornea. Four 3 mm electrodes are connected to the core of the contact lens, which are in turn directly connected to the electrostimulator device.
An anesthetic drop is used before the treatment begins; during stimulation, the patient may feel a small tingling effect on the lids or in the whole eye, but this has not been reported to be painful. The procedure can be performed bilaterally (Figure 3), but when used for the first time, it is better to do only one eye. This is partly because the cables that connect the lens to the device are soft, and if the suction is not correct (which can happen, particularly during the learning curve) they may move, reducing the effect of the treatment and potentially even damaging the corneal epithelium in extreme cases. It also means the patient cannot use their other eye to keep their pupils centered. A speculum can also be used if necessary, which can be helpful to visually ensure that the electrodes are in the ciliary body region, about 3.5 mm from the corneal limbus. After eight minutes of stimulation, the contact lens can be removed (taking care to avoid touching the corneal epithelium), completing the treatment. As the procedure is, in many ways, similar to the application of contact lenses, it should not need to be performed in an operating theater.
Objective challenges
Our initial results were very encouraging, with all the participating subjects showing improvement in accommodation. Using a Jaeger chart, we saw an increase of almost one character – and increases of around one character were also observed using a LogMAR chart at near (40 cm) and intermediate (70 cm) distance (Figure 4). Reading speed also showed improvement – reading times dropped (Figure 5), and more words could be read per minute. Reading from a LogMAR chart in dim light conditions was also improved with treatment (Figure 5). We also measured subjective accommodation amplitude using the Duane test, and found that, on average, patients were able to focus 6 cm closer than before treatment.
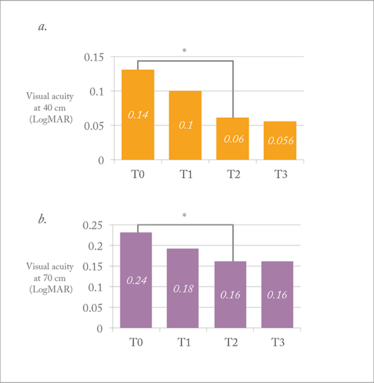
Figure 4. Mean visual acuity (LogMAR) at baseline (T0), treatment 1 (T1), treatment 2 (T2) and treatment 3 (T3) at 40 cm (a) and 70 cm (b). *T2 vs. T0, p<0.05 (Student’s t-test).
As the treatment is based on a passive induction of a stimulus, the exercise, like any stimulation of a muscle, must be repeated to maintain the effect. Based on our early results, we would suggest an “attack dose” of four treatments within two months (one every 10–15 days), and afterwards, to maintain the effect, one treatment every three months – but the frequency of treatments can be customized to the patient’s requirements.
We had our initial results and some positive feedback, but we faced a challenge. How could we demonstrate the effects of the device objectively? This proved difficult – we struggled to find an instrument that could accurately and reproducibly measure the accommodation of the eye. Following a review of the literature, we decided to use ultrasound biomicroscopy (UBM) to study accommodation pre- and post-stimulation, as it allows for visualization of structures in the posterior chamber, and can be used to study the changes that occur during accommodation (see Figure 6). After stimulation, we collected all of this data and found that there was an increase in crystalline lens thickness, a decrease in posterior ray of curvature, and a dramatic decrease in anterior ray of curvature – this translated into a reduction in total spherical aberration. We also examined internal spherical aberration (which is mainly the aberration induced by the lens) and saw a reduction of longitudinal spherical aberration too.
So far, we have compared our anatomical data to both our subjective and objective data, with good results: every significant improvement we saw in patients using subjective data (such as LogMAR and reading speed) corresponded with an improvement in UBM and aberrometry data. We have presented our initial findings at several congresses (4)(5)(6) and now plan to submit our work for publication in a peer-reviewed journal.
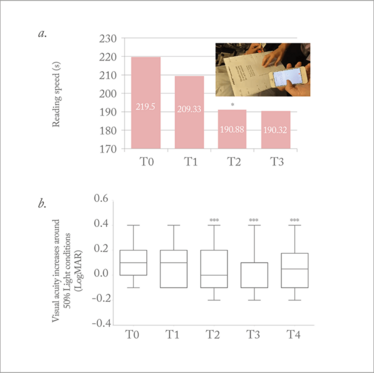
Figure 5. Mean reading speed time (seconds) using MNREAD charts (a) and visual acuity in dim light conditions (b) at baseline (T0), treatment 1 (T1), treatment 2 (T2) and treatment 3 (T3). *T2 vs. T0, p<0.05; *** p<0.001 vs. T0.
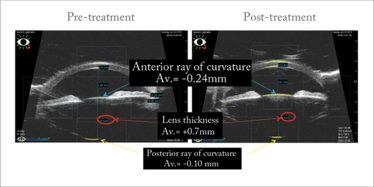
Figure 6. Ultrasound biomicroscopy images (taken under accommodation), obtained pre- and post-stimulation.
Optimization and IOP
When it comes to refractive surgery, choosing the right treatment for a 40 to 50 year-old presbyope can be difficult – and some treatments are more invasive and more permanent than others. Electrostimulation of the ciliary body may provide a new, nonsurgical option to help delay the development of early presbyopia. Through follow up and further study, we hope to confirm the effects we have observed with our electrostimulation approach to date. We also hope to upgrade and optimize our methods (in particular, our stimulation parameters and patterns) in order to try to achieve even better results, both in patients with presbyopia and even in other disease states, such as glaucoma, as previous work has shown that ciliary muscle stimulation can also positively effect IOP (7). In any event, exploring the potential of electrostimulation in ophthalmology could lead to some electrifying advances!
To see a video of the technique, head online to our YouTube channel: http://top.txp.to/LG-ciliary-muscle
Luca Gualdi is a surgeon at the DOMA Eye Clinic, Rome, which specializes in ocular diagnostics, cataract and refractive surgery, and the treatment of keratoconus.
- SJ Garg J Federman, “Optogenetics, visual prosthesis and electrostimulation for retinal dystrophies”, Curr Opin Ophthalmol, 24, 407–414 (2013). PMID: 23799487.
- G Anastassiou et al., “Transpalpebral electrotherapy for dry age-related macular degeneration (AMD): an exploratory trial”, Restor Neurol Neurosci, 31, 571–578 (2013). PMID: 23760223.
- VV Okovitov, “Transconjunctival electrostimulation of eye in pathogenic therapy of progressive myopia”, Vestn Oftalmol, 113, 24–26 (1997). PMID: 9508744.
- L Gualdi, “Microelectrostimulation of the ciliary body as a new noninvasive method for presbyopia treatment: early results”, Paper presented at the American Academy of Ophthalmology; 16 November 2015; Las Vegas, USA. PA042.
- L Gualdi, “Micro-electrostimulation of ciliary muscle to restore accommodation”, Paper presented at the International Society of Presbyopia; 9 September 2016; Copenhagen, Denmark.
- L Gualdi, “Micro-electrostimulation of the ciliary muscle to restore accommodation in early presbyopia”, Paper presented at the American Academy of Ophthalmology; 15 October 2016; Chicago, USA.
- AP Nesterov, EV Khadikova, “Effect of ciliary muscle electrical stimulation on ocular hydrodynamics and vision function in patients with glaucoma”, Vestn Oftalmol, 113, 12–14 (1997). PMID: 9381633.
Luca Gualdi is a surgeon at the DOMA Eye Clinic, Rome, which specializes in ocular diagnostics, cataract and refractive surgery, and the treatment of keratoconus.













