Turning Back Time
Gene therapy has traditionally focused on correcting one defect at a time. But what if you could do more with the tools of the trade? How about reprogramming cells to sidestep disease – or turning back the clock on senescent cells in age-related disease? Here’s what we can do today – and what’s on the horizon.
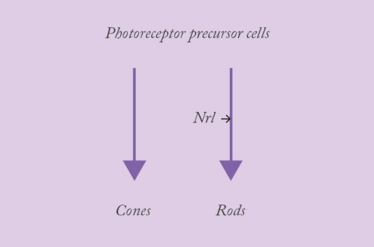
The Neural Retina Leucine Zipper gene (Nrl) plays a fascinating role in retinal development. It encodes a transcription factor that acts as a master control switch for rod photoreceptor differentiation by regulating the expression of important rod-specific genes. These include the rhodopsin-encoding RHO, and PDE6B – a subunit of the cGMP-PDE protein complex, which is essential to light-mediated neurotransmission in the retina. The interplay between the various developmental morphogens and signaling cascades is nuanced and complex… What you really need to know is that all photoreceptor precursor cells develop into cones in the absence of Nrl. But what’s really interesting about Nrl? Its action is reversible. Inactivate it, and rods become reprogrammed and turn into cones.
We’ve known for many years that retinitis pigmentosa (RP) is caused by mutations in genes that are expressed in rods, not cones, and it results in a progressive sequence of rod cell death followed by cone loss. Rod degeneration results in the collapse of the outer nuclear layer (ONL) of the retina, and this generates an oxidative, nutrient-deficient environment that is toxic to cones. It’s actually the cone loss that’s the most debilitating. Think about it: rods are absent from the all-cone fovea centralis, and it’s hard to find an environment where rod-mediated vision is actually used – particularly in urban settings, where artificial light is endemic and most bedside tables have a lamp.
So, if you spare the rod, you save the cone? That’s the conclusion most of us have reached and a number of therapeutic approaches under investigation are trying to achieve this. In some ways, RP is an attractive candidate for gene therapy; the eye is accessible, and efficacy is easily tested through imaging, eletroretinography (ERG) and even standard visual acuity testing, and the genetic defects are well understood. It’s why there are a number of gene therapies currently in Phase I/II clinical trials, with more in preclinical stages. But there are about 200 genes that, if defective, can cause RP – so to treat all RP patients by gene therapy, we would need as many therapies as there are defective genes. Having done an early gene therapy trial (2) on a rare RP condition caused by a Mertk mutation (which has only less than 40 patients in the entire world), and given what we know about Nrl, its inactivation and cell fate, might there be a better way – one treatment to cure them all?
I wanted to answer two big questions: could we make photoreceptor cells insensitive to inherited mutations by reprogramming them such that they switch from a vulnerable to an invulnerable state? And could this – potentially universal – RP therapy work in the clinic to preserve cone-mediated, high-acuity daytime color vision?
The first hurdle that needed to be cleared in taking this approach was a practical one. It’s all well and good using germline techniques to make conditional Nrl knock-out mice as part of an experiment to understand that gene’s function. But that’s not an approach that can be taken in patients. For a clinically relevant approach, you first need a method capable of inactivating Nrl in post-mitotic cells – and these are notoriously resistant to genetic manipulation. Our approach was to use a homology-independent targeted integration (HITI) strategy based on the revolutionary CRISPR/Cas9 technology. The CRISPR/Cas9 guide RNAs are carefully designed to permit precise sequences of host cell DNA to be excised and replaced with transgene sequences, which we deliver to the cells with a vector based on adeno-associated virus (AAV).
In fact, we had already developed an HITI-AAV toolkit and had successfully used it to integrate a transgene (green fluorescent protein) into the DNA of post-mitotic, cultured primary neurons (1). We then went on to use our toolkit on Royal College of Surgeons (RCS) rats, which express a RP-like phenotype (thanks to mutations in the second exon of the Mertk gene). We were able to perform a CRISPR/Cas9-mediated replacement of the mutated gene in three-week-old animals, which not only preserved outer nerve layer (ONL) thickness, but also improved electroretinographic (ERG) responses to light (1), validating a new CRISPR approach for gene therapy.
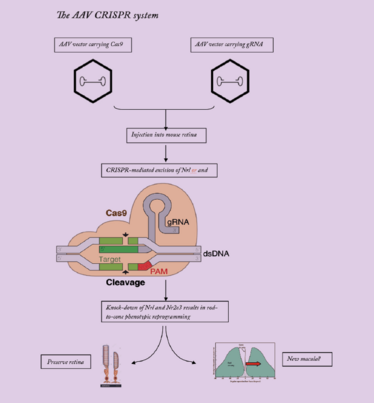
Two AAV vectors are employed, one expressing Cas9, and the other carrying guide RNAs targeting Nrl and Nr2e3. The gRNAS target Cas9 nuclease to genomic Nrl and Nr2e3, resulting in knock-down of these transcription factors.
So, we can successfully transfect post-mitotic photoreceptors with our HITI-AAV system. It’s all great work; however, it isn’t cell-fate switching, nor is it the universal RP treatment I’ve proposed. What it did show was that our CRISPR toolkit could work in patients with RP, and that encouraged us to take the first steps towards using cell-fate switching to treat RP. Now, we have developed an HITI-AAV system that incorporates two guide RNAs targeting Nrl or Nr2e3. You already know why we’re targeting Nrl, so why Nr2e3? It’s a key transcription factor that’s activated by Nrl, and in turn up-regulates the rod-specific gene transcription network – and targeting either or both transcription factors should reprogram rods to cones.
Just like our Mertk work, we first had to demonstrate that the system can switch rods to cones in mice. Long story short: subretinal administration of CRISPR-AAV-nrl at postnatal day 7 in wild-type mice resulted in substantially increased numbers of cone-like cells in the retina (as determined by the presence of the cone cell marker, MCAR), and quantitative PCR demonstrated down-regulation of rod-specific genes and up-regulation of cone-specific genes. The next step was to see if our CRISPR-AAV-nrl system worked across multiple murine RP models with different mutations, so we tested it in Rd10 and FVB/N mice. Again, we saw similar, encouraging results. Treated Rd10 mice showed improvements in vision and photopic b-wave amplitude under ERG testing, suggesting that cone function had improved. The treatment also appeared to preserve cells expressing cone proteins, maintain ONL thickness, and protect S-opsin and PNA expressing cells – cones, in other words. In the FVB/N mouse, treated animals showed preservation of mCAR+ cone cells and maintenance of ONL thickness and, again, displayed improved vision and photopic b-wave values.
So, another hurdle has been cleared: the data effectively show that our CRISPR genome editing approach can reprogram rods to cone-like photoreceptors in post-mitotic tissues, interrupt retinal degeneration and restore visual function – all in at least two different mouse models of RP.
Next, we constructed a CRISPR-AAV-Nrl vector to target the exact same Nrl gene sequence shared by humans and monkeys, which allowed us to use the same AAV vector in both non-human primate (NHP) safety studies and human clinical trials. The preliminary NHP results have been extremely promising: our three-month follow-up data suggest that our approach converts rods to cones in monkeys without any significant toxicity, and we expect to progress to clinical trials later this year.
Seeing the bigger picture
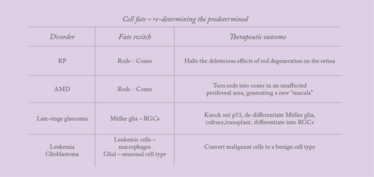
The application of rod to cone cellular reprogramming extends far beyond RP; this approach holds the potential to provide a new treatment option for age-related macular degeneration (AMD). People with advanced AMD suffer progressive vision deterioration and loss due to choroidal neovascularization or geographic atrophy, both of which destroy an increasing proportion of cones in the macula. My proposal is that, given the retina contains 200 million rods and 5 million cones, we should be able to employ a cellular reprogramming strategy to switch a patch of rods into cones in a perifoveal area where retina is unaffected by AMD. This approach could provide end-stage AMD patients with a new “macula” and potentially reverse some of their vision loss. For younger people, however, who are at risk of developing AMD but who have not yet progressed to overt damage, a different approach would be required; for these patients, we are investigating a synthetic biology route (Box 1).
What about people with late-stage glaucoma who have lost a significant proportion of their retinal ganglion cells, compromising their optic nerve? Could we address these situations simply by reprogramming neuroglial cells into retinal neurons? There’s some precedent for this concept. We know that Müller glial cells from the retina of non-mammalian vertebrates produce new retinal neurons in response to injury. And we know that these new cells can structurally and functionally integrate with existing visual circuitry, thereby repairing the retina. Mysteriously, however, mammalian Müller cells appear to have lost this capacity for retinal regeneration. Why?
At last, we have at least one answer: it’s due to p53 function (4). By knocking out p53 in mouse Müller glia, we restore their ability to rapidly and efficiently differentiate into photoreceptor precursors in culture. Further, these Müller glia-derived progenitors can incorporate themselves into the host retina after transplantation, and express markers for either retinal ganglion cells (Islet1 and Brn3) or photoreceptors (rhodopsin and IRBP). Importantly – after all, p53 is a classic tumor-suppressor gene – we observed no tumor formation after transplantation of the precursors.
Reprogramming cells isn’t limited to ophthalmology – there’s a clear rationale for its use in leukemia too. As macrophages are not susceptible to the cancer transformation processes associated with the oncogenic mutations that drive leukemia, we could design a reprogramming strategy that will treat leukemia by switching malignant leukemic cells into benign macrophages. Similarly, cells of glioblastoma multiforme (GBM) – a very aggressive glial cell lineage tumor – could be switched into a neural cell type in which the oncogenes or mutations underlying GBM will no longer be effective.
Box 1: Synthesis of science and hope
What do you offer a young patient at high risk of AMD? Historically, very little – but novel options are now offered by the combination of new synthetic biology techniques and the increasingly detailed understanding of AMD’s genetic basis.
- Nearly 70 percent of AMD cases are associated with just three genes, Htra1/ARMS2, CFH and C3 – why not remove any inherited risk by employing gene-directed approaches?
- One issue is that AMD risk alleles are spread over stretches of DNA comprising hundreds of kilobases – the affected sequences are too long for conventional gene therapy approaches
- Advances in synthetic biology, however, now allow the construction of entire chromosomes, or even – in the case of simple organisms such as yeast – of entire genomes
- By combining gene editing and synthetic biology, it may be possible to excise the entire range of AMD-associated alleles and replace them with normal alleles, in vivo
- Just a few interventions at the genome level would very substantially reduce the AMD risk for an individual.
Remember the vulnerable
It’s clear to me that cellular reprogramming techniques will have a significant therapeutic impact across many disease areas. And as these techniques rely on modulating the patient’s own cells, they are likely to be less risky than modalities based on exogenous stem cells, where there may be a concern of tumorigenesis or immune rejection. Add the fact that reprogramming therapies are not restricted to genetic subtypes and are applicable to very broad range of patients, and we anticipate a broad application of this approach.
That said, there will be big challenges getting these kinds of advanced therapies to everybody who needs them. It’s one thing to develop a technology that can address a medical need – quite another to ensure that it is broadly accessible to patients at low cost. There are still many patients with treatable diseases who go untreated, particularly in rural areas of developing countries. Changing this situation may take decades, and will need a concerted effort by the global community. It will also require the employment of many different tools, not least artificial intelligence, which I believe will have a key role in allowing broad patient access to new therapies (for example, by enabling remote diagnosis via smartphones and the Internet).
Development of simpler and cheaper administration methods also would help: at present, the best way to deliver reprogramming therapy to RPE or photoreceptor cells is to inject vector into the sub-retinal space, but this is costly, inconvenient and requires considerable surgical skill. In the future, however, we hope to administer the treatment as an in-office procedure, just like intravitreal injections (but using robotic devices). And maybe one day, we will be able to eliminate the burden of injections altogether. For example, we’ve been working on a topical eye-drop for wet AMD for the last five years, and expect it to move into clinical trials in the US and China early next year. Replacing intravitreal injections with eye-drops would be of great benefit.

Box 2: Don’t replace – regenerate
Will slow, natural lens regeneration relegate current methods of cataract treatment to the ‘quick and dirty’ category of care?
The Need
- Cataracts are normally treated by replacing the occluded lens with an artificial intraocular lens (IOL)
- This highly successful procedure normally results in improved vision within a few hours
- However, IOL implantation carries a risk of complications, and current IOLs cannot accommodate in the same way as young crystalline lenses
Our Solution
- We developed a method for regenerating a new lens in situ (5), which required:
- development of a cataract removal method that preserves endogenous lens epithelial stem/progenitor cells (LECs)
- identification of factors (Pax6 and Bmi1) required for lens epithelial cells (LECs) to participate in lens regeneration
- Using this method, we regenerated functional lenses in rabbits, macaques and human infants
- The approach may also restore accommodative power, at least where accommodative loss is mainly due to lens sclerosis
What’s Next?
- Modifications to the basic method are in progress:
- Injection of a 3D-printed scaffold, impregnated with appropriate growth factors, is expected to stimulate and guide LECS during lens regeneration
- The intent of this device is to decrease the time required for functional lens regeneration from 6–8 months to 2–3 months, improve refractive outcomes, and allow customization of the lens’ final form to the specific requirements of the patient’s eye
Turning back time is the future
In my research, I always try to consider broad themes that are applicable across different areas of medicine. As a consequence, although I work within ophthalmology, I increasingly find that I am thinking outside the eye field. You could say I’m using ophthalmology as a model to address broad medical needs rather than conditions that are limited to the eye. In particular, one of my overarching goals is to combat age-related diseases – and perhaps to address ageing itself! Indeed, I believe that if we work out how to stop ocular diseases of ageing – such as macular degeneration, glaucoma and cataract – we will also be able to prevent other age-related conditions: Parkinson’s, Alzheimer’s, and so on.
But what’s critical to the development of anti-ageing therapies is achieving a full understanding of the biological clock – we were the first to identify an epigenetic gene modification mechanism that drives the ageing process in every single tissue and cell, including those in the eye. We’ve been working extensively on understanding how this clock ticks – and what makes it tick. In particular, we have been trying to identify and understand the upstream factors that make the clock run faster or slower, and their downstream consequences. Ultimately, this research will enable us to devise treatments that inhibit the ageing process – in fact, we have evidence to suggest that we can even reverse it! I expect – within my lifetime – to see human beings enjoying a longevity of 150–200 years. And this extended lifetime would not be burdened by the frailties of age; centenarians would function as though they were healthy 40–50-year-olds.
Finally, I believe that the best approach to these age-related conditions is to harness the regenerative power contained within our own cells and tissues. I am convinced that the next phase of regenerative medicine will focus on waking up our endogenous stem cells and directing them to repair or regenerate the damaged tissue in situ – another overarching theme of my work. We’ve already seen this happening in cardiac cell regeneration, where you can guide cardiac fibroblasts to differentiate into beating cardiomyocytes; one day, we will see the same principles used to address diseases of old age – for example, to reprogram glial cells into functional neurons as part of an Alzheimer’s treatment. I foresee a wave of innovative therapies in this field over the next ten years – and I hope our technique for regenerating lenses (Box 2) is just the first of them!
- K Suzuki et al., “In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration”, Nature, 540, 144–149 (2016). PMID: 27851729.
- NG Ghazi et al., “Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial”, Hum Genet 135, 327–343 (2016). PMID: 26825853.
- J Zhu et al., “Gene and mutation independent therapy via CRISPR-Cas9 mediated cellular reprogramming in rod photoreceptors”, Cell Res 27, 830–833 (2017). PMID: 28429769.
- JJ Zhao et al., “Induction of retinal progenitors and neurons from mammalian Müller glia under defined conditions”, J Biol Chem, 289, 11945–11951 (2014). PMID: 24523410.
- H Lin et al., “Lens regeneration using endogenous stem cells with gain of visual function”, Nature, 53, 323–328 (2016). PMID: 26958831.
Zhang is Professor of Ophthalmology and Chief of Ophthalmic Genetics at the University of California San Diego, CA, USA. His clinical and research focuses are on novel disease gene targets and treatment, gene and stem cell-based therapies in AMD, diabetic retinopathy, and inherited retinal degeneration. His laboratory uses genetic analyses to gain insights into the molecular mechanisms that underpin macular degeneration and other eye diseases; this knowledge is then used to make genetic changes that either protect the retina from damage, or actively encourage regeneration. Zhang was voted to the 2018 Power List.













