Now You See Them, Now You Don’t
Might temporary microglia depletion treat inflammatory retinal disease?
Microglia are curious cells. They’re the retina’s (and the central nervous system’s) resident macrophages and when infection occurs, they’re the first line of defense. But they’re also central to keeping neurons healthy – in addition to looking for pathogens, they constantly scavenge for plaques and damaged neurons or synapses, always ready to transform into reactive phagocytes. But there’s strong evidence that microglial reactivity is a hallmark of various retinal degenerative and inflammatory diseases, including both rare genetic disorders like retinitis pigmentosa (RP) and X-linked juvenile retinoschisis, and more common multifactorial retinal diseases such as AMD, diabetic retinopathy, glaucoma, and uveitis. Why? It seems that retinal microglia, in disease states, can remove healthy cells too, contributing to vision loss. Studies have shown inhibiting or removing microglia in retinal degenerative disorders can help retain photoreceptors and slow vision loss. But then, they play an important role in maintaining a healthy retina. So, is their inhibition or removal ultimately bad news in the long-term too?
Wai Wong, chief of the US National Eye Institute’s Section on Neuron-Glia Interactions in Retinal Disease, and his team set out to find out more about retinal microglia – and what happens to the retinal if you eliminate them (in mice). To do this, Wong and his team used PLX5622, a drug that blocks the microglial cell survival receptor, CSF-1. Over a period of several days, CSF-1 inhibition strips microglia almost entirely from the retina, leaving just a few cells clustered around the optic nerve. Crucially, the loss of microglia doesn’t affect nerve function. And, as Wong notes, “If we were to get rid of the microglia while a large, inappropriate immune response was happening, we might be able to miss the worst of the inflammation, but still come back into balance at a later point in time. We could hit pause on the immune system in the retina in a directed way.”
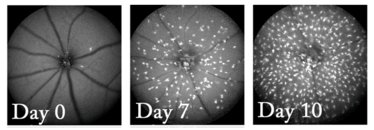
Figure 1. Fundus images of mouse retina after being treated with microglia-eliminating drug, PLX5622. When the drug was stopped (Day 0), nearly all of the microglia had gone, but by Day 7, microglia have started to migrate across the retina, and by day 10 they have increased in number. Credit: Wai T. Wong, National Eye Institute.
What about when you press the play/pause button again? Figure 1 shows the answer. Thirty days after stopping the drug, Wong and his colleagues found that the microglia had repopulated the retina – and by 150 days, they had returned to their normal cell density. “The organization of these immune cells is quite elaborate, and all the organization comes right back,” explains Wong: the returning microglia first started to regrow in clusters the optic nerve head, then gradually, new microglia expanded outwards towards the edges of the retina. Over time, the cells re-established an even distribution across and through the various layers of the retina. As Wong put it: “We can actually image the eye and watch these cells divide and split and migrate as part of the repopulation response.”
But were the new microglia in these mice fully functional? The researchers used a photoreceptor light injury model where photoreceptor cells are damaged by bright light. The new microglia were able to activate and migrate to the injury site normally, and the levels of pro-inflammatory cytokines following light injury were similar in retinas that contained endogenous microglia or repopulated microglia. Electroretinographic testing showed that the repopulated microglia were able to communicate with and fully maintain the function of neurons in the retina (especially when the depletion was short-lived). But significant challenges still remain before this approach can hit the clinic (not least, showing that this approach can actually work in humans). The biggest concern about the clinical use of such drugs is constraining their effect of these microglia-depleting drugs to the retina – as removal of microglia elsewhere in the central nervous system represents maelstrom of potential side effects that are best avoided.
- Y Zhang et al., “Repopulating microglia restore endogenous organization and function under CX3CL1-CX3CR1 regulation”, Science Advances, 4, eaap8492 (2018).
I spent seven years as a medical writer, writing primary and review manuscripts, congress presentations and marketing materials for numerous – and mostly German – pharmaceutical companies. Prior to my adventures in medical communications, I was a Wellcome Trust PhD student at the University of Edinburgh.