
Dry eye disease (DED) is a prevalent ophthalmic condition marked by tear film dysfunction, affecting 9.5-87.5 percent of the global population, with a mean prevalence of 14.6 percent (1). In India, its prevalence ranges from 18.4-54.3 percent (2). DED can develop due to different iatrogenic interventions, including ophthalmic surgical procedures and the use of topical or systemic drugs (3). It is a frequent complication after ocular surgery, mostly following refractive surgeries (48 percent) (4), cataract surgeries (37.4 percent) (5), and eyelid surgeries (26.5 percent) (6), particularly due to the disruption of corneal nerves and altered tear production (7).
Many patients experience DED symptoms immediately after keratorefractive surgery (7). Symptoms typically peak within the first week, gradually improving over 6-12 months, with 8-48 percent of patients continuing to experience issues up to six months post-surgery (7). Following a laser-assisted in situ keratomileusis (LASIK) surgery, DED affects up to 95 percent of patients immediately and 60 percent within one month, a significant concern for corneal refractive surgeons (8). Most patients undergoing refractive surgery in India reportedly show symptoms of iatrogenic DED within 12 weeks (9).
Etiopathogenesis of DED before and after refractive surgery
DED is influenced by intrinsic factors, such as autoimmunity, hormonal imbalances, systemic, and hereditary diseases, nerve damage, and gut dysbiosis, as well as extrinsic factors like environmental influences, behaviors, topical or systemic drugs, and ophthalmic surgeries (3,10). These key factors contribute to ocular surface inflammation and damage, neurosensory abnormalities, hyperosmolarity, and tear film instability (3), which are the core mechanisms of dry eye creation. Figure 1 illustrates the pathophysiology of DED before and after refractive surgery.
Despite the high efficacy and safety of modern corneal refractive surgeries, including LASIK, small incision lenticule extraction (SMILE), and photorefractive keratectomy (PRK), DED remains a concern due to corneal nerve damage during these procedures, as illustrated in Figure 2 (7, 11). It has been reported that corneal nerve injury or transection, leading to a hypoesthetic or anesthetic cornea, is a key pathophysiological change caused by refractive surgeries (7). Loss of corneal sensitivity impairs the corneal-lacrimal reflex loop, impacting tear secretion (7, 11, 13). These procedures also alter the corneal surface, which affects tear film dynamics, leading to uneven tear distribution and rapid evaporation. Additionally, the inflammatory response triggered by surgery can cause meibomian gland dysfunction (MGD), further aggravating DED symptoms (7, 13).
Pathophysiology of preexisting and postoperative DED (3, 8, 11, 12). *The type of surgery, refractive error, and factors, such as the diameter of the lenticule in SMILE procedures and the ablation zone in PRK and LASIK surgeries influence the extent of the damage.
Interestingly, it has been reported that low vitamin D levels negatively impact DED test scores, tear quantity, visual quality of life (QoL), tear film dysfunction, and tear hyperosmolarity, indicating a probable association with DED symptoms (14). Moreover, deficiency of serum vitamin D level is also associated with impaired tear film stability and reduced secretion (14).
Impact of preexisting and postoperative DED on patients who have undergone refractive surgery
Pre-existing DED is common among patients seeking refractive surgery, with approximately 5-60 percent experiencing symptoms, such as fluctuating vision, grittiness, and discomfort, which may not always align with clinical findings (3, 9). Patients are at higher risk of developing more severe postoperative symptoms, including suboptimal visual outcomes, prolonged healing times, and decreased quality of life (QoL) (3, 8). The PROWL study (Patient-Reported Outcomes With LASIK, a prospective, observational cohort study) reported lower satisfaction among patients regarding vision and surgery due to preexisting DED (15). Postoperative DED is one of the most common challenges after refractive surgeries and adversely impacts both vision and QoL (13). The discomfort caused by dryness, such as stinging, pain, photophobia, or visual fatigue triggers dissatisfaction in patients and negatively impacts their perception of successful surgery.
Therefore, routine screening for DED is recommended for all candidates undergoing refractive surgery to improve surgical outcomes (7, 14, 15). This should include an assessment of ocular surface characteristics and symptoms, as well as relevant medical and drug history (15). Since MGD is a major contributor to DED (16), meibography has become an essential component of DED evaluation (13, 16). The impact of different refractive surgeries (LASIK, PRK, and SMILE) on DED symptoms can be evaluated by an altered ocular surface disease index (OSDI) score, reduced corneal sensitivity, and tear-film breakup time (TBUT) (12). Additionally, decreased tear secretion, impaired functional visual acuity or residual refractive errors, and increased symptom scores are observed at one, three, and six months postoperatively (15). Thus, identifying and managing DED preoperatively as well as post-operatively can improve surgical outcomes.
Management strategies for DED
The management of DED aims to alleviate symptoms, restore ocular surface homeostasis, minimize corneal damage, provide a smooth postoperative course, and enhance patient satisfaction (3, 7, 11). This can be accomplished through a step-by-step approach tailored to the severity and type of symptoms. The first step in management includes administering topical lubricants and anti-inflammatory agents (7). Mucin secretagogues and autologous serum can provide further support for managing DED postoperatively (7). Table 1 outlines the perioperative management strategies for DED before and after refractive surgery in patients.
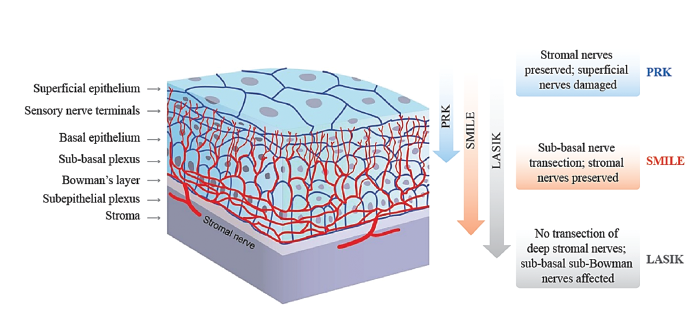
Figure 2. Transection of nerves with different corneal refractive interventions (11, 12).
Table 1. Management strategies for DED before and after refractive surgery.
Table 1. Management strategies for DED before and after refractive surgery.*
Table 1. Management strategies for DED before and after refractive surgery.*
*Common polymers used in artificial tears and their functions:
HA: A naturally occurring lubricant with viscoelastic and hygroscopic properties that provides sustained hydration, increases residence time, reduces tear drainage, enhances tear film stability, and accelerates corneal epithelial wound healing (19).
HP-guar: A mucomimetic and tear-like rheological polymer (gelling agent) mimicking the mucin layer of the tear film, with unique adaptive meshwork that adjusts viscosity and prolongs the lubricant's presence on the ocular surface, improving comfort and vision quality for effective management of DED (19).
CMC: A viscosity-enhancing agent, effective for treating DED, regardless of its origin. (20)
Combination of polymers in artificial tears: Provides superior ocular lubrication, retention, and synergistic benefits compared to single polymer formulations (19-21).
HA + HP-guar: A preservative-free formulation that provides hydration protection against desiccation, greater moisture retention, symptom relief, superior cell barrier function, higher epithelial cell viability, and longer-lasting tissue lubricity compared to HP-guar or HA alone in human corneal epithelial cells (21); it also provides high rates of re-epithelialization compared to HA-containing artificial tears (22).
CMC + HA: Effective and well-tolerated, with greater potential for DED symptom relief compared to CMC alone (20).
According to a survey of Indian ophthalmologists, 61.43 percent believed artificial tears provide continuous relief, 42.86 percent advocated long-term use, and 58.57 percent preferred preservative-free formulations (17).
Abbreviations: CMC, carboxymethylcellulose; HA, hyaluronic acid; HP, hydroxypropyl; HPMC, hydroxypropyl methylcellulose; MMP, matrix metalloproteinases; PEG, polyethylene glycol; PG, propylene glycol.
Table 1. Management strategies for DED before and after refractive surgery.
Table 1. Management strategies for DED before and after refractive surgery.
While proactive management of DED reduces postoperative complications and enhances vision quality, conventional artificial tears often contain preservatives like benzalkonium chloride (BAK) (3), which can significantly harm the ocular surface through direct and indirect cytotoxic effects, leading to dryness, inflammation, discomfort, and delayed hypersensitivity reactions (23). Another preservative known as polyquad, a derivative of BAK, is increasingly used due to its lower toxicity and better antimicrobial efficacy (23). These preservatives are generally better tolerated at low frequency and concentrations, but their safety is influenced by dosage, frequency of use, and individual ocular conditions (23).
Consequently, it has been observed that, for patients recovering from refractive surgery where the ocular surface is already compromised and there is a need for lubricants in higher frequency and for longer duration, preservative-containing artificial tears can hinder healing and prolong symptoms (3,7). Therefore, effective management of postoperative DED using preservative-free artificial tears is the preferred first line of treatment (7). Patients using preservative-free artificial tears report faster symptomatic relief, improved visual quality, and a smoother recovery process (7). It is important, however, to mention that the safety benefits of these eye drops are dependent on the quality of preservative-free packaging to maintain sterility between uses (24).
Conclusion
The high prevalence of DED before and after refractive surgery presents significant challenges, affecting patients’ well-being, QoL, and satisfaction with keratorefractive surgery. Preservative-free artificial tears play a crucial role in postoperative DED management, offering a safe and effective option to support ocular surface healing. This approach improves immediate outcomes and fosters long-term trust in surgical care, leading to happier patients and overall refractive surgery success. Irrespective of the procedure, managing DED ensures a smoother recovery and significantly enhances patient satisfaction.
*Common polymers used in artificial tears and their functions:
HA: A naturally occurring lubricant with viscoelastic and hygroscopic properties that provides sustained hydration, increases residence time, reduces tear drainage, enhances tear film stability, and accelerates corneal epithelial wound healing (19). HP-guar: A mucomimetic and tear-like rheological polymer (gelling agent) mimicking the mucin layer of the tear film, with unique adaptive meshwork that adjusts viscosity and prolongs the lubricant's presence on the ocular surface, improving comfort and vision quality for effective management of DED (19). CMC: A viscosity-enhancing agent, effective for treating DED, regardless of its origin. (20). Combination of polymers in artificial tears: Provides superior ocular lubrication, retention, and synergistic benefits compared to single polymer formulations (19-21). HA + HP-guar: A preservative-free formulation that provides hydration protection against desiccation, greater moisture retention, symptom relief, superior cell barrier function, higher epithelial cell viability, and longer-lasting tissue lubricity compared to HP-guar or HA alone in human corneal epithelial cells (21); it also provides high rates of re-epithelialization compared to HA-containing artificial tears (22). CMC + HA: Effective and well-tolerated, with greater potential for DED symptom relief compared to CMC alone (20).
According to a survey of Indian ophthalmologists, 61.43 percent believed artificial tears provide continuous relief, 42.86 percent advocated long-term use, and 58.57 percent preferred preservative-free formulations (17).
Abbreviations: CMC, carboxymethylcellulose; HA, hyaluronic acid; HP, hydroxypropyl; HPMC, hydroxypropyl methylcellulose; MMP, matrix metalloproteinases; PEG, polyethylene glycol; PG, propylene glycol.
Acknowledgments:
Medical writing support was provided by Priyanka Biswas Karmakar and Somdatta Mukherjee from Turacoz Group.
Disclosures:
Financial support for manuscript writing was provided by Alcon Vision, LLC.
The authors declare no conflicts of interest.
References
- Y Wan et al., “The global prevalence of dry eye disease and its association with economy: a systematic review,” Research Square, July 2019. DOI:10.21203/rs.2.10986/v1
- JS Titiyal et al., “Prevalence and risk factors of dry eye disease in North India: Ocular surface disease index-based cross-sectional hospital study,” Indian J Ophthalmol., 66, 207 (2018). PMID: 29380759.
- K Donaldson et al., “Call to action: treating dry eye disease and setting the foundation for successful surgery,” J Cataract Refract Surg., 48, 623 (2021). PMID: 34694257.
- KS Bower et al., “Chronic dry eye in photorefractive keratectomy and laser in situ keratomileusis: Manifestations, incidence, and predictive factors,” J Cataract Refract Surg., 41, 2624 (2015). PMID: 26796443.
- M Miura et al., “Prevalence and characteristics of dry eye disease after cataract surgery: A systematic review and meta-analysis,” Ophthalmol Ther., 11, 1309 (2022). PMID: 35534685.
- J Prischmann et al., “Dry eye symptoms and chemosis following blepharoplasty: A 10-year retrospective review of 892 cases in a single-surgeon series,” JAMA Facial Plast Surg., 15, 39 (2013). PMID: 23329270.
- S Nair et al., “Refractive surgery and dry eye: An update,” Indian J Ophthalmol., 71, 1105 (2023). PMID: 37026241.
- RM Shtein, “Post-LASIK dry eye,” Expert Rev Ophthalmol., 6, 575 (2011). PMID: 22174730.
- S Dasgupta, R Gupta, “The course of dry eye following phacoemulsification and manual-SICS: A prospective study based on Indian scenario,” Int Eye Sci., 16, 1789 (2016). DOI:10.3980/j.issn.1672-5123.2016.10.0
- Z Al-Mohtaseb et al., "The relationship between dry eye disease and digital screen use,” Clin Ophthalmol., 15, 3811 (2021). PMID: 34531649.
- F Bandeira et al. “Corneal re-innervation following refractive surgery treatments,” Neural Regen Res., 14, 557 (2019). PMID: 30632489.
- B Sharma et al., “Impact of corneal refractive surgery on the precorneal tear film,” Indian J Ophthalmol., 68, 2804 (2020). PMID: 33229655.
- S D’Souza et al., “Algorithmic approach to diagnosis and management of post-refractive surgery dry eye disease,” Indian J Ophthalmol., 68, 2888 (2020). PMID: 33229664.
- J Liu et al., “Vitamin D deficiency is associated with dry eye syndrome: a systematic review and meta-analysis,” Acta Ophthalmol., 98,749 (2020). PMID: 32421222.
- M Eydelman et al., “Symptoms and Satisfaction of Patients in the Patient-Reported Outcomes With Laser In Situ Keratomileusis (PROWL) Studies,” JAMA Ophthalmol. 135, 13 (2017). PMID: 27893066.
- CW Lin et al., “Impact of dry eye disease treatment on patient quality of life,” Front Med (Lausanne), 11, 1 (2024). PMID: 38482530.
- A Shirsat et al., “Prevailing practices for the management of dry eye disease in India: A questionnaire based survey,” IJCEO, 9, 532 (2023). DOI:10.18231/j.ijceo.2023.101
- H Hasani, "Impact of tea tree oil on dry eye syndrome after photorefractive keratectomy laser surgery,” Journal of Iranian Medical Council, 6, 730 (2023). http://dx.doi.org/10.18502/jimc.v6i4.13454
- M Labetoulle et al., “Efficacy and safety of dual-polymer hydroxypropyl guar- and hyaluronic acid-containing lubricant eyedrops for the management of dry-eye disease: a randomized double-masked clinical study,” Clin Ophthalmol., 12, 2499 (2018). PMID: 30584269.
- P Aragona et al., “Safety and efficacy of a preservative-free artificial tear containing carboxymethylcellulose and hyaluronic acid for dry eye disease: A randomized, controlled, multicenter 3-month study,” Clin Ophthalmol., 14, 2951 (2020). PMID: 33061281.
- R Rangarajan et al., “Effects of a hyaluronic acid/hydroxypropyl guar artificial tear solution on protection, recovery, and lubricity in models of corneal epithelium,” J Ocul Pharmacol Ther., 31, 491 (2015). PMID: 26067908.
- E Carlson, “Impact of hyaluronic acid-containing artificial tear products on reepithelialization in an in vivo corneal wound model,” J Ocul Pharmacol Ther., 34, 360 (2018). PMID: 29394128.
- MC Coroi, “Preservatives from the eye drops and the ocular surface,” Rom J Ophthalmol., 59, 2 (2015). PMID: 27373107.
- M Crary, “A review of the containers available for multi-dose preservative-free eye drops,” Biomedical Journal of Scientific & Technical Research,” 45, 36035 (2022). DOI: 10.26717/BJSTR.2022.45.007130
