Multiple Certainties
How can surgeons assure predictable – and optimal – outcomes for cataract patients? Simple: just combine an aberration-resistant IOL with a delivery system that offers unmatched delivery consistency – the 4-in-1 multiSert™ delivery system preloaded with Vivinex™
sponsored by Hoya
Featuring:
Professor Ramin Khoramnia, Associate Professor, Heidelberg University Eye Clinic, Germany.
Professor Susana Marcos, Professor of Research and Head of the Visual Optics and Biophotonics Laboratory, Institute of Optics, National Center of Research, Madrid, Spain.
A Unique Combination of Clarity and Control
Modern ophthalmology benefits from an extraordinary arsenal of techniques and instruments. Today’s standard for cataract surgery: small-incision surgery followed by the delivery of a foldable IOL, using a sterile disposable injector. The approach is not only convenient and simple, but also avoids direct handling of the IOL, thereby keeping it undamaged and sterile throughout the procedure.
But not all IOLs are equal – and nor are their delivery systems. For example, with standard IOLs the image quality can be affected by their spherical surface, which adds to the positive spherical aberration intrinsic to the human cornea. Aspheric IOLs were developed to avoid this issue: in these lenses, the absence of spherical surfaces eliminates IOL-related positive spherical aberration. The next step was to design IOLs with negative spherical aberration that actually corrects for the positive aberration of the cornea, so that the net aberration of the eye’s optics moves closer to zero. In effect, these more advanced IOLs mimic the normal optical interplay between cornea and crystalline lens – and thereby push image quality closer to that typical of a young eye.
Improved IOL performance, however, can be a double-edged sword: more advanced lenses may be more susceptible to other forms of aberration. In particular, lenses with negative spherical aberration are associated with coma; all it takes is a little decentration for such IOLs to induce significant coma (1)(2)(3), and it is important to note that off-axis conditions occur naturally in every eye (1).
Traditional negative aspheric IOLs must therefore be very precisely and stably positioned, as misalignment will compromise the retinal image (1)(2)(3) – and this in turn places greater demands on the delivery device. The ideal injector would offer the surgeon the opportunity to modulate lens insertion according to preference and clinical need, but currently available devices offer limited flexibility. Furthermore, many injectors can deliver the IOL inconsistently from case to case, sometimes requiring the surgeon to adjust their technique accordingly; inconsistent folding, straight, looped, or sticking haptics, stretched incisions, sub-optimal insertion depths and sudden lens release are just some of the unpredictable issues that surgeons experience in the OR.
Recognizing these problems, HOYA Surgical Optics has developed the 4-in-1 multiSert™ preloaded injector system, an entirely new IOL delivery system that provides greater flexibility and control during IOL delivery. This novel device is preloaded with HOYA’s market-proven Vivinex™ IOL – a lens with unique optics that resist coma and reduce spherical aberration, thereby providing superior image quality (1). This combination – which has now received CE marking in Europe – is the latest phase in HOYA’s ambitions to make IOL insertion more precise and predictable, and to provide cataract patients with unprecedented clarity of vision. What kind of assurances can surgeons take from this first of its kind injector-IOL combination?
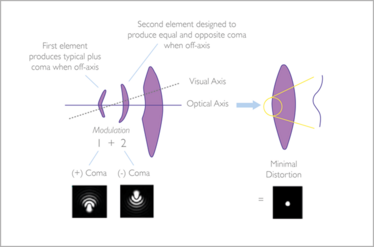
Figure 1: Independent optical elements 1 and 2 in the Vivinex™ IOL induce positive and negative coma, thereby canceling out coma caused by the naturally occurring off-axis conditions in the eye (7).
Vivinex™: Unprecedented clarity of vision in the presence of natural off-axis placement
The unique benefits of the 4-in-1 multiSert™ delivery system preloaded with Vivinex™ arise partly from the intrinsic features of Vivinex™ itself. The lens is fabricated from a novel hydrophobic acrylic, and uses a proprietary manufacturing process with a unique, active oxygen posterior surface treatment step. The result? A lens designed to significantly reduce posterior capsule opacification (PCO) (4)(5), and which is glistening-free; accelerated in vitro glistening evaluations have established that Vivinex™ meets Grade 0 glistening criteria on the Miyata scale (6).
We might expect these characteristics alone to provide unprecedented clarity of vision, but Vivinex™ also offers another unique feature; its optical design provides a lens that is more tolerant to coma, caused by the naturally occurring off-axis conditions found in the eye, than traditional negative spherical aberration IOLs. In other words, Vivinex™’s innovative aspheric optics reduce spherical aberration without incurring significant susceptibility to decentration-associated coma (1).
Vivinex™: Unique optical design
Briefly, the patented design of the Vivinex™ IOL includes two distinct aspheric elements in the center of the IOL which work in harmony (7). The central 1.2 mm optical zone induces positive coma by using a convex meniscus structure, while the 1.2 mm area around this central zone induces an equal negative coma through a concave meniscus structure (Figure 1). Thus, the lens as a whole is designed to cancel out coma, providing patients with improved off-axis image quality versus traditional negative aspheric IOL designs (7).
What does this mean for surgeons and patients? Quite simply, Vivinex™ not only provides -0.18 μm of spherical aberration correction, but also is more tolerant to coma caused by the natural off-axis conditions that occur in the eye (Figure 2). Furthermore, the mechanism for coma tolerance is rotationally symmetrical, and its efficacy is unaffected by the exact direction of the visual axis after decentration. Therefore, the very precise positioning constraints that generally apply to other negative aspherical IOL designs do not apply to the Vivinex™ IOL (1).
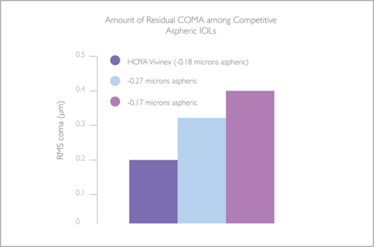
Figure 2: Residual coma is less in HOYA Vivinex™ in the presence of decentration as compared with other leading competitor IOLs. (RMS: root mean square) (1).
The Vivinex™IOL: providing peace of mind
This means Vivinex™ has the unique ability to compensate for variations in the relative position of the IOL versus the visual axis, and assures almost aberration-free vision over a wide field; no other IOL can give surgeons this certainty and peace of mind. And in terms of Vivinex™’s performance, the numbers speak for themselves (see The Vivinex™ Advantage).
The logic behind the Vivinex™ design is clear: but are the theoretical benefits supported by independent research? One recent study stands out: an investigation into the effect of off-axis conditions, or decentration, on image quality in different aspheric IOL designs (Vivinex™, HOYA, -0.18 µm aspheric correction, n=10; Tecnis 1P ZCB00V, Johnson and Johnson Vision, -0.27 µm aspheric correction, n=4; AcrySof 1P SN60WF, Alcon, -0.17 µm aspheric correction, n=4). Each IOL was mounted in a model eye that mimics the anatomical parameters of the pseudophakic eye, and optical image quality (as represented by modulation transfer function [MTF] and visual Strehl ratio) was evaluated at lateral decentrations of zero, 0.4 mm and 0.7 mm. At all decentrations, Vivinex™ had visual Strehl and MTF values superior to other IOLs (Table 1) (1).
These data suggest that lateral decentration is associated with decreased retinal image quality – including coma – and that of the three IOLs tested, Vivinex™ is most immune to this effect.
Professor Susana Marcos, senior author on the study, explains the findings and their impact in Box 1.
BOX 1 : Effects of IOL decentration on image quality
Susana Marcos is Professor of Research and Head of the Visual Optics and Biophotonics Laboratory at the Institute of Optics, National Center of Research, Madrid, Spain. Her multidisciplinary group develops new technologies for eye imaging and vision correction, and has generated 17 patents in this field.
Why is it important to investigate IOL susceptibility to decentration-induced aberrations? Remember that the eye is intrinsically non-centered, partly due to the eccentric position of the fovea – yet it provides good image quality across the visual field, and is relatively immune to decentration. IOLs that incorporate and optimize a higher number of variables in a more sophisticated optics design may be able to mimic this property. We wanted to investigate this by comparing decentration-associated aberrations in different commercially available IOLs. We employed a water-cell model eye incorporating a reflective retina, an aspheric cornea with realistic spherical aberration, and a 3-axis micrometer stage holder that allows mounting and fine displacement of the IOLs (1). A custom-developed laser ray tracing aberrometer was used to measure optical aberration at different levels of IOL decentration; MTFs were computed from these measurements.
Primarily we found that not all IOLs are equally susceptible to decentration-induced astigmatism and coma – so not all will maintain equivalent optical quality upon decentration. In particular, our results indicated that the Vivinex™ IOL is less susceptible to decentration than other IOLs. These data are significant because they demonstrate that the Vivinex™ design works as intended. Given that the eye is intrinsically decentered, new IOL designs that provide stable optical performance under decentration are of clear interest. Furthermore, it is likely that more sophisticated IOLs – such as toric or multifocal designs – will also benefit from technology that resists decentration-induced image degradation. For each design, the relative benefits would need to be confirmed in model eyes, as in our study, and ultimately in more advanced models based on patient-specific data. In all cases, due regard should be given to the relative contributions of lens design, ocular surfaces and alignment.
Over the years, IOLs have evolved from simple spherical designs to more sophisticated products based on optimization of multiple variables. This journey has, in parallel, improved both the understanding of the optics of the crystalline lens and of the whole eye – a consequence of advances in quantitative imaging and optical characterization. The reported work exemplifies the benefits of this continuous feedback between basic research and lens design.
Delivering results: the 4-in-1 multiSert™ injector system
In reality, providing an aberration-resistant IOL is only half the battle; it still needs to be safely and conveniently delivered to the capsular bag. Existing IOL delivery devices take the ‘one size fits all’ approach: surgeons must work with the device as it is, regardless of their preferred technique or of particular clinical demand – hardly reflective of real-world complexity. Therefore, HOYA developed the 4-in-1 multiSert™ injector (Figure 3) to provide an entirely new option in IOL delivery: a device that not only offers different operating modalities to fit the diverse techniques and preferences of different surgeons, but also meets different clinical needs. Specifically, multiSert™ offers two key innovations: the ability to switch between single-handed push and two-handed screw implantation modes, as well as the ability to control penetration depth and protect wound integrity through a uniquely-designed ‘insert shield’ (Figure 4). Together, these advances allow the surgeon to fine-tune the cataract procedure – offering unmatched control during IOL implantation.
The Vivinex™ Advantage
Optical performance confirmed by respected scientists
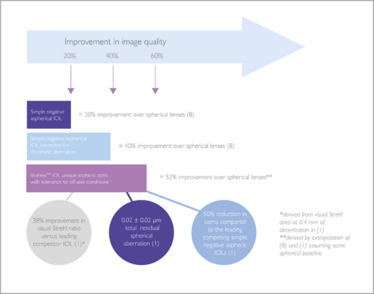
The Vivinex™ Advantage. Based on data showing improvement in modulation transfer function: 5 degree visual axis, 4 mm pupil diameter (3).
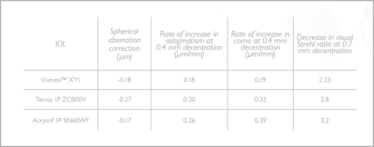
Table 1: Visual Strehl and MTF values are superior in Vivinex™, as compared with competitor IOLs, at all tested decentration values (1).
Table 1: Resistance to decentration-induced aberration in different IOLs
Reassuringly flexible
The multiSert™ gives surgeons the option of using a two-handed ‘screw’ or a one-handed ‘push’ mode during implantation. The push mode works similarly to a standard syringe – the lens is moved forward simply by advancing the multiSert™ plunger, and in doing so the IOL is automatically folded with the IOL haptics in a consistent configuration. This ensures a highly reproducible intraocular delivery experience for the surgeon (10).
Control of penetration depth is achieved by the afore-mentioned insert shield which is positioned around the injector cartridge. The end of the shield forms a stop that limits the insertion of the injector tip into the eye – and if the surgeon prefers it can be moved closer to the injector tip to limit the length of the injector tip that may enter the eye (Figure 4). Thus, surgeons may select depths compatible with IOL implantation either directly into the capsular bag or through the incision tunnel, according to their personal preference.
Proven performance
How does the multiSert™ perform? During in vitro studies in the OR environment and ex vivo wet lab investigations, participants reported that multiSert™ gave outstanding performance in delivery and release of the Vivinex™ IOL (10) (Table 2). In vitro assessments of multiSert™ safety, usability and acceptability were performed in the ORs of 14 European clinics (four sites in Austria; three in France; and seven in Germany). In each site, assessment was conducted by one surgeon and one scrub technician; 16 injectors were tested per site, making a total of 224 separate evaluations. Users reported their experiences via questionnaire (each question was scored on a scale of 1–5: 1=poor; 2=fair; 3=average; 4=good; 5=excellent). In push mode, the multiSert™ was reported to be easy to use, and the surgeons verified that both leading and trailing IOL haptics were correctly tucked in 100 percent of cases. After IOL delivery, none of the injector tips were found to be broken when used according to manufacturer’s directions. Further notable findings from these assessments are provided in Table 2.
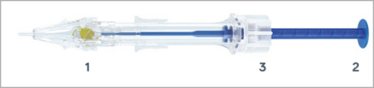
Figure 3: Note: 1, the insert shield positioned on the outside of the cartridge, to control penetration depth; 2, the plunger for push mode; and 3, the rotational knob for screw mode.
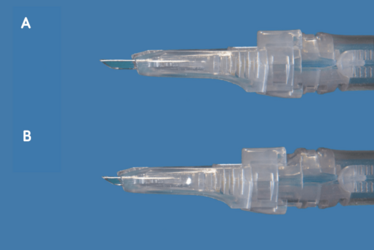
Figure 4: The insert shield may be retracted (A), to allow deeper penetration, or advanced (B) to limit penetration depth.
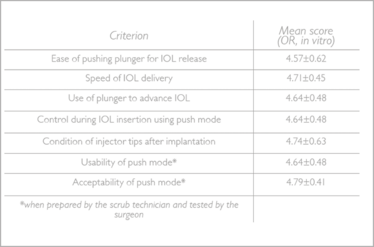
Table 2: Selected data from independent assessment of multiSert™ performance (10). Each criterion was rated on a 5 point scale, with 5 indicating the highest satisfaction level. The multiSert™ systems were preloaded with Vivinex™ IOL model XY1 (yellow) or XC1 (clear), of refractive power low (6 D), medium (20 D) or high (29 D); both model and power were randomized.
Table 2: Summary of usability assessment data
But what about clinical use of the multiSert™ preloaded delivery system with Vivinex™? Professor Ramin Khoramnia describes his first experiences with the system (Box 2).
Box 2: Clinical use of multiSert™
Ramin Khoramnia is Associate Professor at the Heidelberg University Eye Clinic, Germany. He undertakes 10 or more cataract operations per day, with a high proportion of difficult cases: eyes with small pupils, pseudoexfoliation syndrome, hard cataracts and poor visibility.
What preloaded IOL delivery systems do you use? How is multiSert™ different from these?
I frequently use iSert® (HOYA), AutonoMe (Alcon), iTec (Johnson and Johnson Vision), BLUEMIXS (ZEISS), and RaySert (Rayner) systems. The multiSert™ differs from these primarily in that surgeons can choose between two modes of lens delivery (push or screw) in a single device. Furthermore, the modes can be easily changed during implantation (from push to screw or vice versa). This flexibility of operation makes for a very versatile delivery system that is compatible with a broad range of surgical techniques.
The multiSert™ also has a unique insert shield, which limits the depth of penetration; when might this be useful?
Shallow penetration assists in cases where the wound requires protection – for example, to avoid surgically-induced astigmatism, or where the cornea in the incision region is thin. Conversely, deeper penetration facilitates implantation directly into the bag.
What is your experience of multiSert™?
So far, I have used the multiSert™ in five OR procedures and several wet lab procedures. I’ve found that multiSert™ preparation is very comparable to that of Vivinex™ iSert® – and just as easy. I have never had a case in which the iSert® has been prepared incorrectly, and I would expect the same with Vivinex™ multiSert®: it is almost impossible to make a mistake during device preparation, or to ‘load’ the lens incorrectly. Similarly, lens delivery with multiSert™ is as consistent as with iSert® – the haptics are always in the right position. Like the iSert®, the injector tip has been designed for very small incisions, and therefore enters the wound very easily. Furthermore, the multiSert™ has a useful safety feature: the lens power is printed on the injector, allowing final verification prior to implantation.
What practical advantages does multiSert™ bring?
The option of using either screw or push modes is intrinsically advantageous in ORs shared by surgeons with different IOL delivery preferences – the multiSert™ “fits” all surgeons because of its versatility. Also, the ability to change between modes during implantation is very useful in procedures that are not straightforward.
Additionally, the ‘push’ mode frees up one of the hands that would otherwise be employed in the two-handed ‘screw’ technique. This hand can then help manipulate the lens using another instrument, via the side port. Furthermore, the ‘push’ option permits either slow or rapid lens insertion; the latter saves time in routine cases and increases efficiency. The screw mode is particularly good in eyes requiring a very slow release of the lens – for example, in instances of zonular weakness. A great feature of the multiSert™ is that it allows surgeons to switch from push to screw in these cases.
In both modes, the lens is delivered in a very controlled fashion. Such control is important, because push delivery systems have not always been compatible with hydrophobic IOLs and some have been associated with unpredictable lens release. With multiSert™, however, lens delivery is controlled and predictable even in push mode. Also, the IOL advance can be stopped at any point during implantation – essential for
difficult cases.
Final thoughts?
HOYA has done a great job with multiSert™. It is very compatible with my preferred technique, and most surgeons will be able to adapt the injector to their specific needs by choosing from its large range of settings. Vivinex™ can be very safely and easily implanted with the new device – it is very pleasing that an excellent IOL (glistening-free hydrophobic acrylic and advanced optics) is combined with a simple, predictable implantation system.
An unbeatable combination of clarity and control
The combination of the Vivinex™ IOL with the new multiSert™ 4-in-1 preloaded delivery system brings the best of both worlds – a high quality IOL platform and a uniquely flexible delivery system. Not only does the Vivinex™ IOL exploit advanced materials and proprietary manufacturing processes, but it also features an innovative optical design that tolerates coma caused by the naturally occurring off-axis conditions that occur in the eye. The number of eyes that have received Vivinex™ IOLs – more than 1 million sold – is testament to the unprecedented clarity of vision associated with HOYA’s unique IOL. But this popularity also reflects an increasing recognition that IOLs cannot be perfectly aligned with the visual axis in routine cataract surgery, and that surgeons should consider the potential for decentration-related effects in even the most straightforward of procedures. In this context, the advantages of a lens that tolerates decentration-induced coma are clear.
Furthermore, the European CE marking (9) of the multiSert™ delivery system preloaded with Vivinex™ gives surgeons access to not only the most advanced IOL of its type, but also to a dual mode push/screw delivery system that offers unmatched levels of flexibility, precision and control during lens insertion. The innovative multiSert™ insert shield provides additional assurance – operators can modulate the insertion depth according to preference, and therefore implant Vivinex™ either directly into the capsular bag or via the incision tunnel: no other IOL delivery system offers these features.
HOYA’s highly novel IOL-injector combination will be presented at the 36th Congress of the European Society of Cataract and Refractive Surgeons (ESCRS; 22–26 September 2018, Vienna, Austria), and will be commercially available in the range of 6 to 30 Diopters – in both clear and yellow lenses – in selected countries from September 2018 onwards.
HOYA Surgical Optics names and logos are registered trademarks of HOYA Surgical Optics, Inc. © 2018 HOYA Surgical Optics, Inc. All rights reserved. Tecnis and AcrySof are trademarks of their respective manufacturers.
>> Download pdf
- P Pérez-Merino and S Marcos, “Effect of intraocular lens decentration on image quality tested in a custom model eye”, J Cataract Refract Surg, 44, 889–896 (2018). PMID: 30055694.
- J Tabernero et al., “Predicting the optical performance of eyes implanted with IOLs to correct spherical aberration”, Invest Ophthalmol Vis Sci 47,4651-4658 (2006). PMID: 17003464.
- A Harrer et al., “Variability in angle kappa and its influence on higher-order aberrations in pseudophakic eyes”, J Cataract Refract Surg, 43, 1015-1019 (2017). PMID: 28917399.
- H Matsushima et al., “Active oxygen processing for acrylic IOL to prevent posterior capsule opacification”, J Cataract Refract Surg, 32, 1035-40 (2006). PMID: 16814067.
- MA Farukhi et al., “Evaluation of uveal and capsule biocompatability of a single piece hydrophobic acrylic IOL with UV ozone treatment on the posterior surface”, J Cataract Refract Surg, 41, 1081-1087 (2015). PMID: 25935337.
- P Merz, “IOL optic material purity study”, performed by David J Apple International Laboratory for Ocular Pathology, University of Heidelberg, and Institute of Applied Mathematics, University of Heidelberg (2015).
- D Sanger et al., “Intraocular lens”, Vivinex™ US patent 8647383 B2 (2014).
- HA Weeber and PA Piers, “Theoretical performance of intraocular lenses correcting both spherical and chromatic aberration”, J Refract Surg, 28, 48-52 (2012). PMID: 22074466.
- HOYA Surgical Optics. “HOYA achieves CE mark for first-of-its kind 4-in-1 multiSert™™ preloaded delivery system with Vivinex™™ intraocular lens (IOL)”. Available at: bit.ly/multiViv. Accessed: July 4, 2018.
- D Nogueira, “Usability and acceptability evaluation of the multiSert™ injector system”, HOYA data file DoF-SERT-102-MULT-03052018 (2018).