Enter the Exosome
Simpler to produce and easier to administer than stem cell therapies – are exosomes the answer to our retinal regeneration needs?
Ben Mead |
At a Glance
- Until recently, exosomes were thought to function only as waste product excretion vehicles; in fact, they contain functional molecules that can alter the phenotype of non-cognate cells
- In particular, the ocular neuroprotective effect of stem cells – which depends on preservation of existing neurons rather than on neuroregeneration – is mediated by stem cell exosomes
- Current work demonstrates this effect in the optic crush model, where exosome-vectored microRNAs eliminate about two-thirds of the RGC death seen in untreated animals
- Stem cell-derived exosomes may form the basis for a novel, cell-free neuroprotective glaucoma therapy
Retinal stem cell therapy works – but the cells aren’t really working in the way you were promised they would back in college. It turns out that they protect the retina not by differentiating into and replacing damaged neurons, but by rescuing existing, compromised cells. But how?
Ten years ago, few would have guessed that exosomes – small extracellular vesicles that are known to assist the elimination of the by-products of cellular metabolism – had any function beyond waste management. But today, the evidence suggests that these humble structures have significant and beneficial effects in terminally differentiated neural tissues.
Waste not
The surge of interest in exosomes over the last decade was triggered by the demonstration that exosomes contain functional mRNA and microRNA (1). Furthermore, exosomes can mediate transport of these RNAs to entirely different cell types where the exogenous messenger is translated into protein, and the microRNAs modulate endogenous gene expression. In other words, exosomes enable one cell to modulate the protein phenotype of another.
After this (unprecedented) observation, researchers began to postulate functional roles for exosomes. One hypothesis was that malignancy-derived exosomes may contribute to malignant transformation of previously healthy cells, but a happier hypothesis was also made: the therapeutic potential of exosomes. In heart disease, for example, the administration of mesenchymal stem cell-derived exosomes mediates a cardioprotective effect similar to that of the stem cells themselves. Why use a cell therapy approach – with all the potential issues of unwanted proliferation, differentiation (and de-differentiation) and migration – when secreted factors give the same effect? This rationale was behind our decision to investigate the use of isolated exosomes for retinal therapy.
Ways and means
Cells expel many extracellular vesicles; the first step in developing an exosome therapy is to remove all unwanted vesicles. Fortunately, this is simply a question of ultracentrifugation. In brief, we expand stem cells in a vat and harvest the culture medium; spin it at low speed to remove cell debris; spin at high speed to pellet the exosomes, and then wash off the supernatant. This simplicity is one of the great advantages of the exosome approach – it’s so much easier than isolating specific proteins from cell culture medium. Another advantage is the stability of the preparation: unlike cells, which must be maintained at 37°C with appropriate nutrition, exosomes are stable at -20°C for extended periods (up to a year, according to some reports). Furthermore, they are easier to administer than cells: dense cell suspensions are too viscous to easily inject, which constrains the number of cells you can administer. Exosomes, by contrast, can be easily injected in huge numbers. Finally, it’s easy to get robustly quantifiable data with exosomes, whereas cell delivery counts require guesswork – the vitreous isn’t a great substrate for stem cells, and cell survival rates are unclear, even a few hours after injection.
But does it work?
We decided to investigate the therapeutic potential of stem cell exosomes in the optic nerve crush model (2). This a good model to test neuroprotective strategies for retinal ganglion cells (RGCs): traumatizing the optic nerve (with a crush) destroys RGCs within about three weeks while leaving all other retinal cell types intact. In our study, we administered intravitreal injections of 3×109 exosomes at the same time as the crush. Three weeks later, we sacrificed the animals, retrieved the retinal tissue and stained the cells for microscopy. When we compared the number of RGCs surviving in treated and untreated animals, we saw a clear difference: untreated rats lost about 90 percent of their RGCs, while treated rats lost only about 30 percent (Figures 1 and 2). Furthermore, the exosome-treated RGCs maintained function as measured by electroretinography (Figure 3).
We also investigated the mechanism by which exosomes might mediate this pronounced protective effect, and found that down-regulating stem cell microRNA results in exosomes that displayed a reduced protective effect (Figure 2). This finding strongly suggests that the great majority of exosome-mediated neuroprotection can be attributed to their microRNA component.
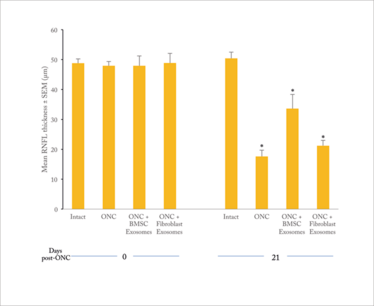
Figure 1. RNFL thickness in rat eye after optic nerve crush (ONC) and exosome treatment (2).
Note that ONC eyes lose well over half of the RNFL thickness; in BMSC exosome-treated eyes, the loss is reduced by about 50 percent. Fibroblast exosomes confer much lower protection.
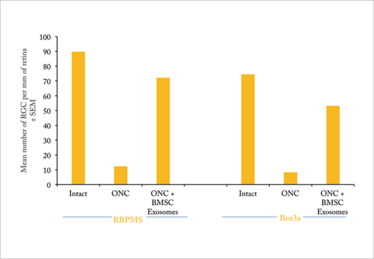
Figure 2. Mean number of RGC per mm of retina in rat eye after optic nerve crush (ONC) and exosome treatment. Note that the ONC results in loss of approximately 85 percent of RGCs in untreated rats, but rats treated with MSC exosomes lose only about 20 percent of their RGCs. This protective effect is largely eliminated by using exosomes from stem cells in which microRNA production has been downregulated (2).
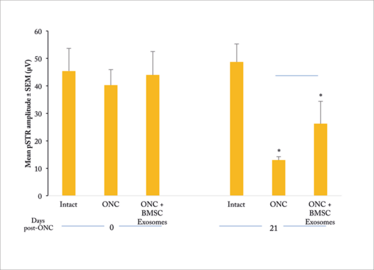
Figure 3. Positive scotopic threshold response (pSTR) in treated and untreated rats after ONC (2). ONC results in a ~70 percent reduction in mean pSTR amplitude in untreated rats; exosome treatment cuts this loss of function by about half.
Time to translate?
Our work indicates that stem cell exosomes may represent the basis of an entirely new therapeutic modality: one that uses stem cells not as a tissue replacement product, but as a source of sub-cellular protective factors that mediate their effect through the target cells themselves. Exosomes have similarities to viral and liposomal gene therapy vectors – but are far easier to produce. They are also extremely straightforward to administer: intravitreal injection is already standard clinical practice and is already received by millions of patients a year.
There are other reasons to believe that the clinical pathway for exosome-based glaucoma therapies will be straightforward. In particular, stem cell therapies are already in trials for the treatment of retinal disorders, including glaucoma and optic nerve injury – for example, the SCOTS trial (3), which is testing autologous bone marrow stem cells in retinal and optic nerve damage and disease. Given that these cells are secreting exosomes, you could say that exosomes are already in the clinic! All we are proposing is a more efficient way of delivering the vesicles – one that avoids the risks, such as retinal detachment, associated with ocular cell therapies.
A possible issue is the fact that exosomes contain thousands of different microRNAs, which in theory could modulate thousands of different genes – a potential source of off-target effects. But again, the same could be said of whole cell therapies, and so far, there are no data to suggest that these have safety issues related to exosome activity. Maybe in the future, we will identify the microRNA subset responsible for the exosome therapeutic effect, and build a therapy based on that alone. That said, it may be simpler to use naturally occurring vesicles, especially given that they are so easy to isolate, store and administer.
Looking ahead, we envisage exosome-mediated therapy not just for glaucoma but also for conditions, such as AMD. Identifying all possible applications will require us to address various fundamental questions, such as optimal dosing and required dosing frequency. And that means there’s plenty to keep us busy!
Ben Mead received his PhD from the University of Birmingham (UK) for work on the use of stem cells to treat retinal disease and to prevent the death of retinal ganglion cells. Currently, he is a post-doctoral fellow at the Section of Retinal Ganglion Cell Biology of the National Eye Institute, Bethesda, MD, USA, where he is investigating the neuroprotective potential of stem cell factors. His current position is funded by the Marie Skłodowska-Curie Fellowship Scheme
- H Valadi, et al., “Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells”, Nat Cell Biol, 9, 654–659 (2007). PMID: 17486113
- B Mead and S Tomarev, “Bone marrow-derived mesenchymal stem cell-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms”, Stem Cells Translational Medicine, 6, 1273–1285 (2017). PMID: 28198592
- clinicaltrials.gov/ct2/show/NCT01920867
Ben Mead received his PhD from the University of Birmingham (UK) for work on the use of stem cells to treat retinal disease and to prevent the death of retinal ganglion cells. Currently, he is a post-doctoral fellow at the Section of Retinal Ganglion Cell Biology of the National Eye Institute, Bethesda, MD, USA, where he is investigating the neuroprotective potential of stem cell factors. His current position is funded by the Marie Skłodowska-Curie Fellowship Scheme.













