Challenging Convention
Why Fuchs endothelial dystrophy might not be a “dystrophy” at all…
At a Glance
- Corneal transplant surgery has seen multiple refinements in the last 25 years – whereas Fuchs endothelial dystrophy (FED) used to be treated with full thickness PKs, now DMEK is the technique of choice
- Surprisingly, partial DMEK graft detachment can lead to great outcomes, suggesting that the descemetorhexis component of DMEK surgery might promote endothelial corneal regeneration
- Although current data on isolated descemetorhexis (without donor tissue) is controversial, several studies are investigating whether this approach is sufficient to achieve corneal clearance in patients with FED
- Certain genetic variants impact on the regenerative capability of corneal endothelial cells and lead to FED. Future therapies might be tailored according to the regenerative potential of these cells
For over a century, ophthalmologists have been able to treat corneal endothelial dysfunction with some form of keratoplasty. But for the majority of that period, that form was full-thickness penetrating keratoplasty (PK) – even in diseases with clearly localized dysfunction like Fuchs endothelial dystrophy (FED) and bullous keratopathy. The concept of lamellar keratoplasty has been around since the 1950s, thanks to the work of legendary corneal surgeons like Joaquín Barraquer and Charles W. Tillet III. But it wasn’t until the late 1990s, when the Dutch ophthalmologist Gerrit Melles described and performed the first successful procedure: posterior lamellar keratoplasty (PLK) (1)(2)(3)(4)(5). Melles’ technique was adopted in the United States by Mark Terry, who termed the procedure “deep lamellar endothelial keratoplasty” (DLEK) (6)(7).
The advantages of PLK over PK are many. Relative to PK, PLK results in considerably less postoperative change in refractive power, induces far less astigmatism, has significantly lower risks of suture-related complications, a lower risk of late wound dehiscence, and even the postoperative burden of continuing care is less (8)(9)(10)(11). There was only one drawback... the technical difficulty of the procedure: it required surgeons to manually dissect both donor and host stromal beds.
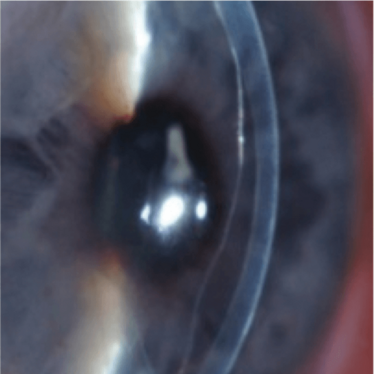
Figure 1. Clear cornea despite an almost
completely detached DMEK graft.
The beginnings of an idea…
By 2003, Melles and his colleagues from the Netherlands Institute for Innovative Ocular Surgery (NIIOS) had come to the belief that “carving out” a posterior lenticule – composed of stroma and Descemet membrane – from the recipient cornea was unnecessary. Instead, it seemed sufficient to merely strip away the diseased Descemet membrane and endothelium (a process they dubbed “descemetorhexis”). The impact at the time was hard to underestimate: using the same corneal donor tissue as used in PLK, surgeons could perform keratoplasty – termed Descemet Stripping Endothelial Keratoplasty (DSEK) – in a manner that was considerably simpler, faster and easier to perform than PLK ever was (1)(12)(13). The adoption of this new endothelial keratoplasty procedure was aided by the use of microkeratome predissection of donor tissue (first described by Mark Gorovoy), which led to the terminology being modified to DSAEK: Descemet stripping automated endothelial keratoplasty (14).
This was refined by Melles et al. in 2008 to create Descemet membrane endothelial keratoplasty (DMEK), in which the graft is “thinned down” into a tissue comprised exclusively of an isolated layer of Descemet membrane and endothelium – without donor stroma. This meant that DMEK achieved an exact, one-to-one replacement of patients’ diseased Descemet membrane with donor tissue. Of all of the techniques described so far, DMEK gives the fastest visual recovery, the highest level of visual acuity postoperatively and the lowest rejection rate of all endothelial keratoplasty techniques (15).
But once again, this advance came at a cost, as DMEK is more difficult to perform than its predecessors – in terms of both tissue preparation and the surgical procedure itself. Unlike DSAEK surgery, the donor tissue is not shaped like a lenticule, but more like a cigar roll. This is due to the elastic properties of the Descemet membrane and the fact that the tissue is only around 20 μm thin – and these properties might explain the most frequent complication during and after DMEK surgery: graft detachment. Reported detachment rates vary from 4 to 73 percent (16)(17), which seems like a huge variation until you understand that some surgeons reserve the term “detachment” for clinically significant events (such as those that undermine the patient’s vision or require some form of reintervention), whereas others describe a graft as having “detached,” even if the location of non-adherence is small, peripheral, and clinically inconsequential.
Going against convention
Some of the most striking improvements in visual outcomes after endothelial keratoplasty have, ironically, been observed in eyes with partially detached DMEK grafts. In 2009, Melles et al. (18) described unexpected corneal clearance with visual recovery up to 20/28 (0.7) and 20/20 (1.0) in two DMEK-treated eyes that showed (near) complete graft detachment in the early postoperative phase (Figure 1, Figure 2a–c). Slit-lamp observation showed cellular repopulation of the host posterior stroma in the presence of a clearly detached graft. Both corneas also cleared from the periphery towards the center, and this observation had important implications; it suggested that endothelial migration might occur as a wound healing response following keratoplasty surgery, and a response that results in the redistribution of the endothelial cells across the posterior cornea.
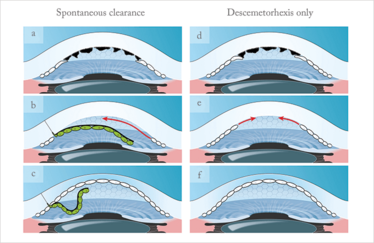
Figure 2. a-c. Possible spontaneous clearance explanation: after descemetorhexis (removal of the physical barrier) the donor somehow induces endothelial cell migration from the periphery towards the center. Consequently, the posterior bare stroma gets covered by endothelial cells that clear up the cornea;
d-f. Theory of isolated descemetorhexis, i.e. without any donor tissue. After removing the guttae, which might act as a barrier, peripheral stem-like cells are able to migrate again towards the center and re-endothelialize the posterior bare stroma.
This was controversial. It flew in the face of conventional wisdom that the host endothelium was incapable of regeneration, and challenged the entire concept of a Fuchs endothelial “dystrophy” and indeed, the necessity for a “keratoplasty.” These findings also raised important questions. Do endothelial cells have regenerative capabilities? Do we still need donor tissue? Could we simplify the surgical procedures to treat endothelial disorders?
Potential answers to these questions can be found in a study we published in 2012 – the first series of so-called DMET (Descemet membrane endothelial transfer) procedures (19). The idea of DMET was based on the observation of corneal clearance, despite partial graft detachment (Figure 1). Because we did not observe any corneal clearance in eyes with complete graft detachment (i.e. a free floating graft in the anterior chamber), we hypothesized that a minimal contact of the graft to the posterior stroma is mandatory.
In search of answers
Our DMET trials were performed in the following manner: after a descemetorhexis, the DMEK graft was injected in the anterior chamber and fixated at the interior lip of the clear corneal tunnel – ending in what was basically a large graft “detachment” and a “denuded” central stromal area. In total 12 eyes were operated upon, seven from patients with FED and five from patients with bullous keratopathy. The results really surprised us.
All eyes operated on for Fuchs showed progressive corneal clearance – clearing completely after 3–6 months (Figure 3). Specular microscopy showed that the endothelial cells were visible and that the pachymetry values has returned to normal. However, not a single eye operated on for bullous keratopathy exhibited corneal clearance, and no endothelial cells were visible by specular microscopy.
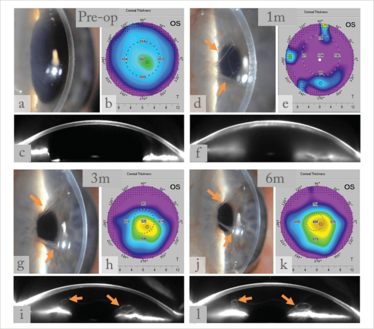
Figure 3. Collage of slit-lamp pictures, pachymetry maps, and Scheimpflug images before (a-c) and 1, 3 and 6 months (d-l) after DMET surgery. a-c, preoperative pictures of a cornea with FED; d-f, almost complete graft detachment one month postoperatively, decompensated cornea; g-i, still a large detached graft, but progressive corneal clearance at 3 months postoperatively; j-l, the graft remained in the same position, but the cornea cleared up with pachymetry levels down to normal.
To explain these results, we hypothesized that, in patients with bullous keratopathy, nearly the entire pool of recipient endothelial cells had been wiped out, whereas in patients with Fuchs, the endothelial cells were merely in some inhibited or arrested state and were (at least potentially) capable of rebounding. Furthermore, the difference in clinical outcomes between the patients with Fuchs and bullous keratopathy may indicate that the recipient – not primarily the donor – endothelium is principally involved in restoring corneal clearance. If so, then this may indicate that endothelial cells in patients with Fuchs are not really “dystrophic” per se, but somehow “dormant” instead (Figure 4d-h).
Guttae are tiny drop-like outgrowths in the corneal endothelium seen in the early stages of FED, and may cause visual impairment, and the endothelial cells that cover these posterior extensions exhibit “thinning.” Endothelial thinning may result in a compromised barrier function, or an increased cell surface area exceeding the endothelial cell pump capacity, or both. The consequence is secondary edema.
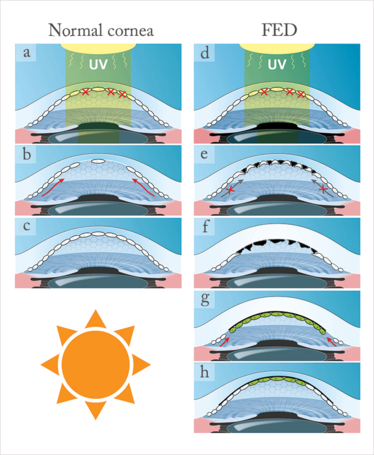
Figure 4. a–c. Possible wound healing response in a normal cornea after apoptosis induced by (for example) UV radiation. However, in corneas with FED (d), the central endothelial cells are even more susceptible to UV-induced damage (thinnest area of the cornea), resulting in a higher number of apoptotic cells and more gaps between cells. Here, the defect cannot be covered by the peripheral stem-like cells because of the physical barrier in the form of guttae (black structures) (e,f). Removing this possible physical barrier (guttae) may open door for recipient and donor cells to migrate and mix and keep the cornea clear after DMEK (g,h).
If the term “dystrophy” is reconsidered for this condition first recognized by Ernst Fuchs (without doubting his seminal findings), it would open the door to also reconsidering its surgical management. If visual impairment is primarily attributable to the presence of guttae (i.e., a Descemet membrane-related disorder), the surgical treatment may also be directed towards removing the Descemet membrane and its guttae rather than transplanting donor endothelium.
Could the answer really be this easy? Just remove the Descemet membrane and trust the regenerative capabilities of host endothelial cells to treat the Fuchs endothelial “disease” (Figure 2d-f)? To answer this question, we have to go deeper into the Fuchs pathophysiology (20).
Digging deeper still
Although not much is known about the pathological mechanisms that underlie FED, it’s suspected that both genetic mutations and environmental factors (21)(22) can underlie the disease. Gene mutations have been found in both inherited and even some sporadic presentations, but this represents a tiny proportion of cases: in most circumstances, FED arises because of an impaired defense to environmental factors like oxidative stress – particularly oxidative stress that’s secondary to ultraviolet radiation, and it appears that people with impaired oxidative DNA damage repair pathways are particularly susceptible to the disease (Figure 4d; 23, 24). This phenomenon might explain why the disorder manifests first in the corneal center, as this is typically the region where oxidative stress is most prominent.
It appears that the endothelial cell layer of the human cornea may have limited regenerative capacity (25–27), but a recent study (28) suggested that the corneal periphery contains a reservoir of stem-like cells that replace damaged endothelium by continuous centripetal migration (Figure 4a–c). These stem-like cells are supposedly protected from environmental oxidative stress-induced damage, precisely because they are located at the very edge of the cornea. However, in FED, this wound healing mechanism might be blocked by the presence of guttae, which act as a physical barrier to the centripetal migration of these stem-like cells (Figure 4e). Might this explain the clinical observations above that suggested that recipient endothelial cells migrated after DMEK?
If we assume that removing the physical barrier (guttae) with a penetrating or endothelial keratoplasty enables the peripheral stem-like cells to migrate again and to mix among the donor cells, this means that descemetorhexis in DMEK surgery “opens the door” for healthy endothelial cells to migrate towards the center of the cornea (Figure 2d–f). This might also explain the faster clearance over the gap between the edge of the descemetorhexis and the edge of the transplant, than over the transplant itself (29) (Figure 5), due to the closer access to the peripheral cornea support zone. This theory is supported by the results of our latest endothelial keratoplasty technique, “Hemi-DMEK” (30–32), which involves tissue bisection after descemetorhexis to create two half-moon shaped grafts for transplantation (Figure 6). Despite large areas of “denuded” posterior stroma, corneas that receive the Hemi-DMEK grafts exhibit clear corneas six months after surgery (30–32; Figure 5).
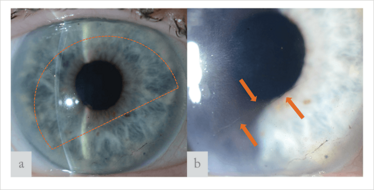
Figure 5. (a) Slit-lamp images of a cornea six months after Hemi-DMEK. (a) Dotted line displays the position of the Hemi-DMEK graft. (b) Arrows display the inferior edge of the graft. The large “denuded” gap between edge of the descemetorhexis and the Hemi-DMEK graft is covered with endothelial cells and shows corneal clearance.
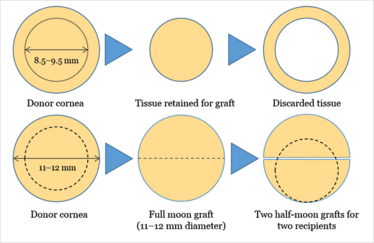
Figure 6. Current preparation techniques aim to harvest the central part of Descemet membrane and endothelium (8.5–9.5 mm). But as the Descemet membrane graft is very thin, there’s no technical or optical reason to only utilize the central portion of the donor tissue. Half-moon shaped “Hemi-DMEK” grafts reduces wastage and provides two DMEK grafts from a single donor cornea. Dashed line circle represents a standard 9.5 mm-diameter DMEK for comparison.
A game-changer in the making?
If our theory is correct, it means we will all have to reconsider our current approach of managing FED with keratoplasty, irrespective of the genetic or environmental cause. It also raises the question of whether we still need donor tissue, or if an isolated descemetorhexis (without implanting any donor tissue) might be sufficient to achieve corneal clearance (Figure 2d–f). On this issue, only controversial data has been published to date (33)(34), but to my knowledge, some isolated descemetorhexis studies are pending publication and are currently yielding promising results. If this concept proves to be successful, it could minimize surgical intervention, its possible complications, eliminate the issues of graft rejection, graft failure, and certainly ease the issue of donor tissue shortage. It’s possible that the different genetic disorders that underlie some Fuchs cases might result in different regenerative capacities of the stem-like cells in the corneal limbus, so a tailored approach might be required.
It might very well be that different genetic variants of FED show different regenerative capabilities so that in future tailored minimal invasive treatment options may be developed based on genetic analysis in the treatment of Fuchs endothelial “dystrophy.”
Martin Dirisamer is a Cornea consultant at the Department of Ophthalmology, University Munich (LMU), Germany and Co-owner of the Smile Eyes refractive laser clinic in Linz, Austria
- GR Melles et al., Cornea, 17, 618–626, (1998). PMID: 9820943.
- GR Melles et al., Am J Ophthalmol, 127, 340–341, (1999). PMID: 10088746.
- GR Melles et al., Ophthalmol, 107, 1850–1856, (2000). PMID: 11013184.
- GR Melles, F Lander, Cornea, 21, 325–327, (2002). PMID: 11917186.
- GR Melles, N Kamminga, Ophthalmologe, 100, 689–695, (2003). PMID: 14504892.
- MA Terry, PJ Ousley, Cornea, 20, 239–243, (2001). PMID: 11322409.
- MA Terry, PJ Ousley, Cornea, 24, 59–65, (2005). PMID: 15604868.
- S Jain, DT Azar, Curr Opin Ophthalmol, 12, 262–268, (2001). PMID: 11507339.
- JL Alio et al., Curr Opin Ophthalmol, 13, 224–229, (2002). PMID: 12165704.
- GI Duncker et al., Klin Monbl Augenheilkd, 221, 14–23 (2004). PMID: 14745673.
- G Geerling et al., Ophthalmologe, 102, 1140–1148 (2005). PMID: 16283187.
- GR Melles et al., Cornea, 23, 286–288, (2004). PMID: 15084862.
- FW Price Jr, MO Price, J Refract Surg, 21, 339–345, (2005). PMID: 16128330.
- MS Gorovoy, Cornea, 25, 886–889, (2006). PMID: 17102661.
- M Rodríguez-Calvo-de-Mora et al., Ophthalmology, 122, 464–470, (2015). PMID: 25439596.
- C Monnereau et al., JAMA Ophthalmol, 132, 1192–1198, (2014). PMID: 24993643.
- K Laaser et al., Am J Ophthalmol, 154, 47–55, (2012). PMID: 22465365.
- C Balachandran et al., Am J Ophthalmol, 148, 227–234, (2009). PMID: 19442962.
- M Dirisamer et al., Am J Ophthalmol, 154, 290–296, (2012). PMID: 22633346.
- M Bruinsma et al., Eye (Lond), 27, 1115–1122, (2013). PMID: 23846374.
- T Schmedt et al., Exp Eye Res, 95, 24–34, (2012). PMID: 21855542.
- H Elhalis et al., Ocul Surf, 8, 173–184, (2010). PMID: 20964980.
- R Buddi et al., J Histochem Cytochem, 50, 341–351, (2002). PMID: 11850437.
- P Czarny et al., Mol Biol Rep, 40, 2977–2983, (2013). PMID: 23275192.
- WF Treffers et al., Ophthalmol, 89, 605–613, (1982). PMID: 6181449.
- VP Hoppenreijs et al., Surv Ophthalmol, 2, 155–164, (1996). PMID: 8890441.
- EG Olsen, M Davanger, Acta Ophthalmol, 41, 885–892, (1984). PMID: 6524313.
- Z He et al., Stem Cells, 30, 2523–2534, (2012). PMID: 22949402.
- M Dirisamer et al., Am J Ophthalmol, 152, 543–555, (2011). PMID: 21726849.
- FC Lam et al., Graefes Arch Clin Exp Ophthalmol, 253, 1955–1958, (2015). PMID: 26156680.
- FC Lam et al., JAMA Ophthalmol, 132, 1469–1473, (2014). PMID: 25211529.
- N Gerber-Hollbach et al., Br J Ophthalmol, [Epub ahead of print], (2016). PMID: 26837507.
- G Moloney et al., Can J Ophthalmol., 50, 68–72, (2015). PMID: 25677286.
- SB Koenig, Cornea, 34, 1149–1151, (2015). PMID: 26186374.
Martin Dirisamer is a cornea consultant at the Department of Ophthalmology, University Munich (LMU), Germany and Co-owner of the Smile Eyes refractive laser clinic in Linz, Austria.













