
Testing Times for Gene Therapies
We are ready for gene therapy, but the accessibility and streamlining of gene testing needs to be improved
At a Glance
- Gene therapy in ophthalmology is starting to come of age, with the first clinical agent having now reached Phase III trials
- But before therapy can even be administered, gene testing has to be performed first. Today, this process is still cumbersome, confusing and time-consuming
- As ever-more genes (like the Wnt gene family) are uncovered that can cause retinal disease, the need for gene testing is only going to increase
- The entire process needs to be streamlined by reducing the costs involved, and enabling patients to access them more easily
My personal interest in gene therapies goes back to my PhD, when I worked on a gene therapy using recombinant adeno-associated virus (AAV) for autosomal dominant retinitis pigmentosa (RP). This was a really exciting time in molecular research as it was around the first time that people were really looking at ways to utilize viruses for therapeutic purposes, rather than trying to figure out how to combat them. After my PhD, I completed my fellowship in Michigan at Associated Retinal Consultants, the same group where Albert Maguire had completed his fellowship. Albert and his wife Jean Bennett received their funding to start RPE65 gene therapy treatment for Leber Congenital Amaurosis (LCA) at this same institution (1). Excitingly, this involved the same viral vector that I had worked on as a graduate student. During my fellowship I was lucky enough to be part of a huge pediatric retina group, and the more I studied the Wnt gene family, the clearer it became that Wnts play a very important role in normal retinal development and maintenance. It struck me that if we could understand how they work and what they do in the retina, we could modulate their function as therapy – and that is my big focus: I study Wnt signaling and look at how this can be manipulated to develop effective gene therapies for Wnt-associated vitreoretinopathies.
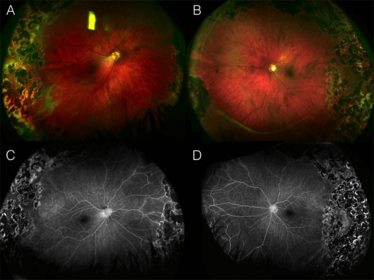
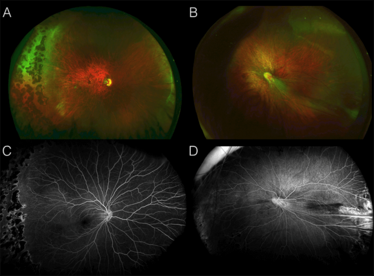
Color wide-field and fluorescein angiography images of fundi from two siblings with Wnt-associated vitreoretinopathy. The patients both have mutations in the frizzled-4-receptor (FZD4) gene. The resultant C181Y mutation in the N-terminal extracellular domain of FZD4 may affect binding of Wnt and signaling.
Getting it into the clinic
I believe that there will come a time when tailored genetic interventions to fix genetic mutations for the treatment of retinal diseases will be commonplace. Gene therapy – at least for the eye – has mostly been performed with AAV vectors, and today we have gene therapies for retinal disease in late-stage clinical trials: RPE65 gene transformation for LCA and RP, and there are two ongoing clinical trials evaluating the use of RS1 as replacement therapy for congenital X-linked retinoschisis (2,3).
When it comes to gene therapies in the clinic, my “pie in the sky” ideal scenario is one where the patient comes into the clinic, we perform a genome screen to figure out the problem, and then tailor the treatment to them. Why is this “pie-in-the-sky?”
Because today, the biggest thing holding back the advance of gene therapies into routine clinical practice is gene testing, which for many, is cumbersome, confusing and time-consuming, for reasons which I will now explain.
In my opinion, although the majority of ophthalmologists appear excited by the potential of gene therapies, they don’t want to be burdened with gene testing at this point, and this definitely has something to do with the ease and efficiency of being able to order genetic testing. Here in the US, if you ask the vast majority of clinicians whether they perform gene testing, they will say, “No I don’t. One, it is cumbersome to my practice. Two, it is not going to change how I manage my patients.” This needs to change. Gene therapy and gene testing go hand-in-hand; once we have gene therapy we will have to have a way to expedite gene testing. For this system to work, we need the process of gene testing to become streamlined, and for this system to not be cost-prohibitive as it is to some extent now.
Jumping through hoops to obtain a test
I am blessed to be a research-based ophthalmologist, as I can perform a lot of gene testing in-house. But this is also a curse because when I’m not able to do so, there’s a multitude of obstacles that get in the way and hoops that have to be jumped through, just to do a test. Patients have to go down the pathway of seeing multiple physicians and getting authorization from their insurance providers. Once the patient reaches the right physician, the physician has to take time out, go online and find a laboratory that will perform the required test, and then find out the logistical and methodological aspects of getting it done – all alongside preparing strong clinical documentation supporting the need for testing. Going down this pathway means that a lot of patients – I would estimate around four in every five – are lost.
With genetic testing, reluctance can also be an issue when patients and parents sense that there are going to be difficulties or obstacles to getting their result. Although you will always have those few patients who may be reluctant to undergo gene testing due to the “fear” of finding out something is wrong, or concern over what it will do to their insurance premiums, the vast majority of patients – at least here in the US – do want to undergo testing: in my clinic, I’ve never had a parent who did not want their children to be tested for Wnt or Wnt-associated mutations. The “want” is there, we just need to overcome the difficulties and obstacles to
genetic testing.
Overcoming obstacles
But how can we overcome these difficulties? The cost of gene panel testing – whether it is covered by insurance, a health service or the patient – needs to be low enough for a patient to afford it. Going back 15 years, the high costs were somewhat justifiable because of the lack of testing facilities and technologies available, but now, we have so many new ways to do genetic analysis that high-throughput total genomic screening is possible: cost shouldn’t be a barrier anymore. Sample collection also needs to be accessible. In an ideal world, when a patient comes in saying “I have night blindness,” you should be able to grab a cheek swab, or a blood sample, and send it to a large central hub that performs the tests and returns the information. Now, I don’t believe that it’s possible for every institution to perform their own genetic testing for all genetic diseases: diagnostics centers are needed. We – and our technicians – should be easily able to obtain a sample and send it to a central location with a simple instruction such as “LCA panel” without delay or causing a backlog in the clinic.
So what should an ophthalmologist who wants to maximize the amount of gene testing they perform do? My recommendation depends on the situation: the hospital’s configuration, location and available resources. Physicians and ophthalmologists in a University or hospital setting often have access to a strong genetics department: the pipeline for testing is there, and essentially, all that is needed is to strike up a rapport and maintain a good working relationship. Those in private practices may have to be more proactive, and collaborate with other colleagues to facilitate the required authorizations. The current dogma is basically to find somebody else who specializes in that the area and go to them. However, there are several helpful (if under-promoted) resources available to help with access to gene testing. The NIH’s Genetic Testing Registry website (4) is one example: you simply put in the gene of interest and the website directs you towards laboratories who can perform that testing.
Looking to the future
Cataloging patients is also a key resource that I think should be more commonplace: there is so much to learn, and we as physicians do not take enough advantage of this. My group has a biobank: I set it up in 2003, and after only a couple of years, we had such volumes of data that we could start to see patterns emerge, and start making sense of the data. And the data started to guide our treatment and management of patients. The data from the biobank led to a number of research studies, and five years ago, we opened the Pediatric Research Retina laboratory at Oakland University. Our biobank now has samples from over a 1,000 children with various Wnt gene family mutations, and we have a number of ongoing projects including researching oxygen-induced retinopathy and neuroprotection of retinal cells. I currently hold patents that relate to different ways of manipulating Wnt signaling, enabling us to modulate neuroprotection, angiogenesis, inflammation, and even perform diagnostic procedures, and so far our preclinical data have been promising. Our hope is that we will be able to start a Phase I trial in the next year looking at Wnt-associated vitreoretinopathies. Although the trials are aimed at obtaining key safety data, we anticipate that we will get some clues regarding efficacy too. Looking to the future, our central aim is to manage children and young adults with vitreoretinopathies, but we are hoping that our work will eventually have a wider applicability. Currently, large volumes of patients with these retinopathies fall under the radar; these diseases are considered to be rare and “big pharma” just isn’t interested unless there is potential to expand it into something larger. If we show that we can manage capillary dropout, promote angiogenesis, and prevent neurodegeneration with gene therapy, we will be opening the doors to much broader therapeutic spectrums, including therapies for diabetic changes and vein occlusions.
Kimberly Drenser is a Consultant Ophthalmologist at Associated Retinal Consultants and is the Director of the Pediatric Retinal Disease Molecular Genetics Laboratory and Director of Ophthalmic Research at Beaumont Eye Institute in Royal Oak, Michigan, USA. Kimberly is also a consultant and a member of the data safety and monitoring board at Spark Therapeutics.
- AM Maguire et al., “Safety and efficacy of gene transfer for Leber’s congenital amaurosis”, N Engl J Med, 358, 2240–2248 (2008). PMID: 18441370.
- Clinicaltrials.gov, “Study of RS1 Ocular Gene Transfer for X-linked Retinoschisis”, (2016). Available at: clinicaltrials.gov/ct2/show/NCT02317887. Accessed September 26, 2016.
- Clinicaltrials.gov, “Safety and Efficacy of rAAV-hRS1 in Patients With X-linked Retinoschisis (XLRS)”, (2016). Available at: clinicaltrials.gov/ct2/show/NCT02416622. Accessed September 26, 2016.
- GTR, “Genomic Testing Registry”, (2016). Available at: www.ncbi.nlm.nih.gov/gtr/. Accessed September 26, 2016.
Kimberly Drenser is a Consultant Ophthalmologist at Associated Retinal Consultants and is the Director of the Pediatric Retinal Disease Molecular Genetics Laboratory and Director of Ophthalmic Research at Beaumont Eye Institute in Royal Oak, Michigan, USA. Kimberly is also a consultant and a member of the data safety and monitoring board at Spark Therapeutics.













