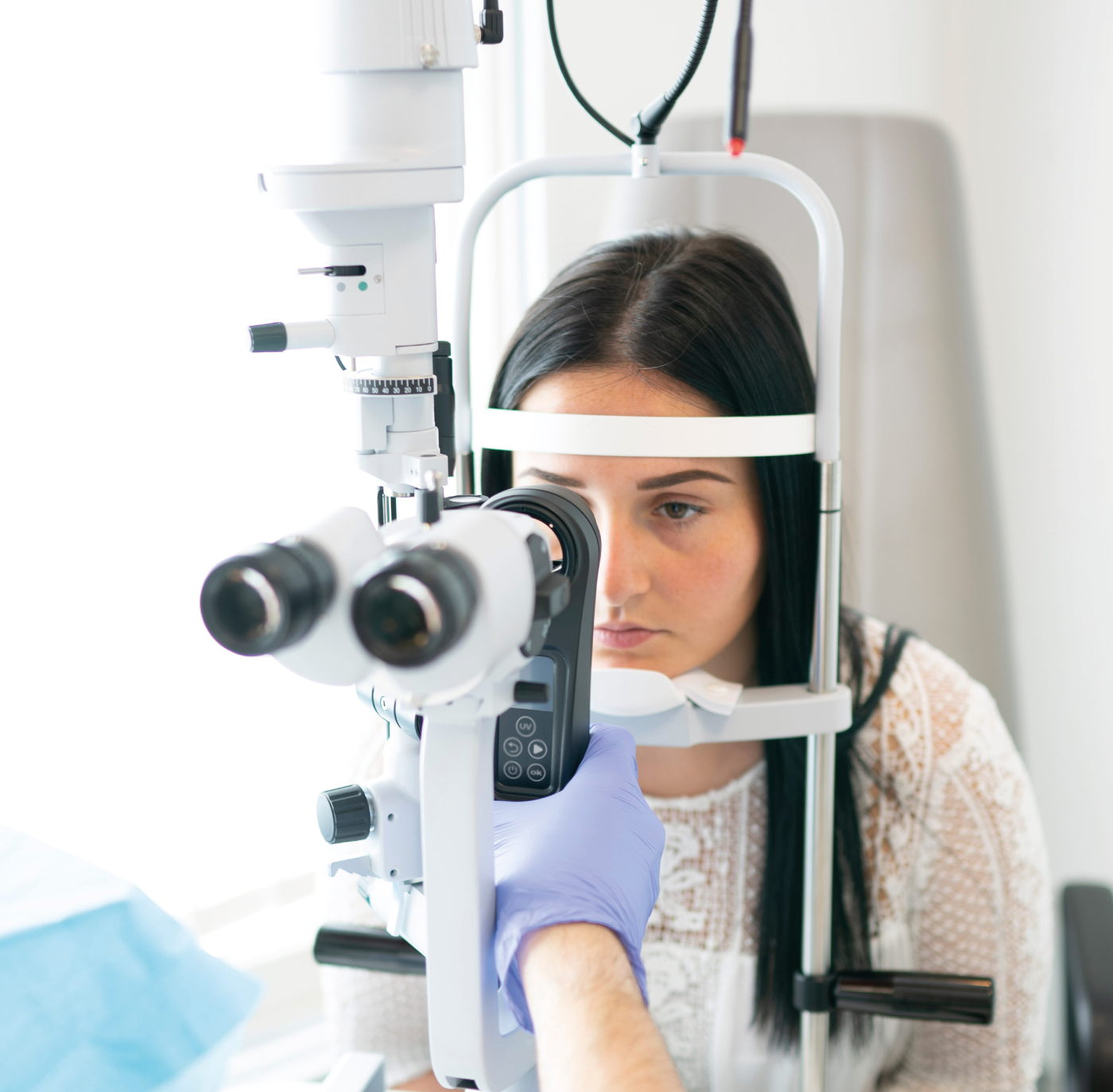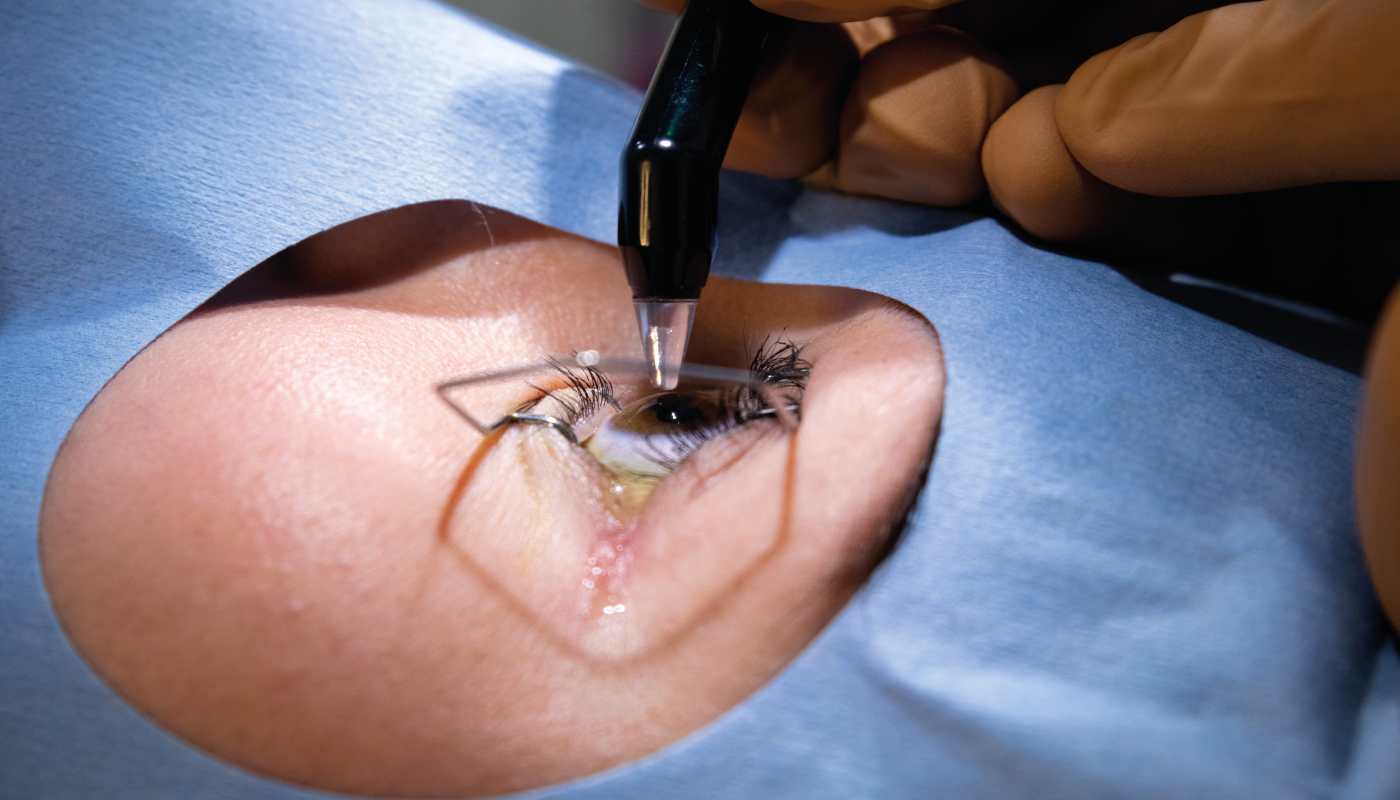
Here is the first part of this story...
We’ve secured regulatory approval… or have we?
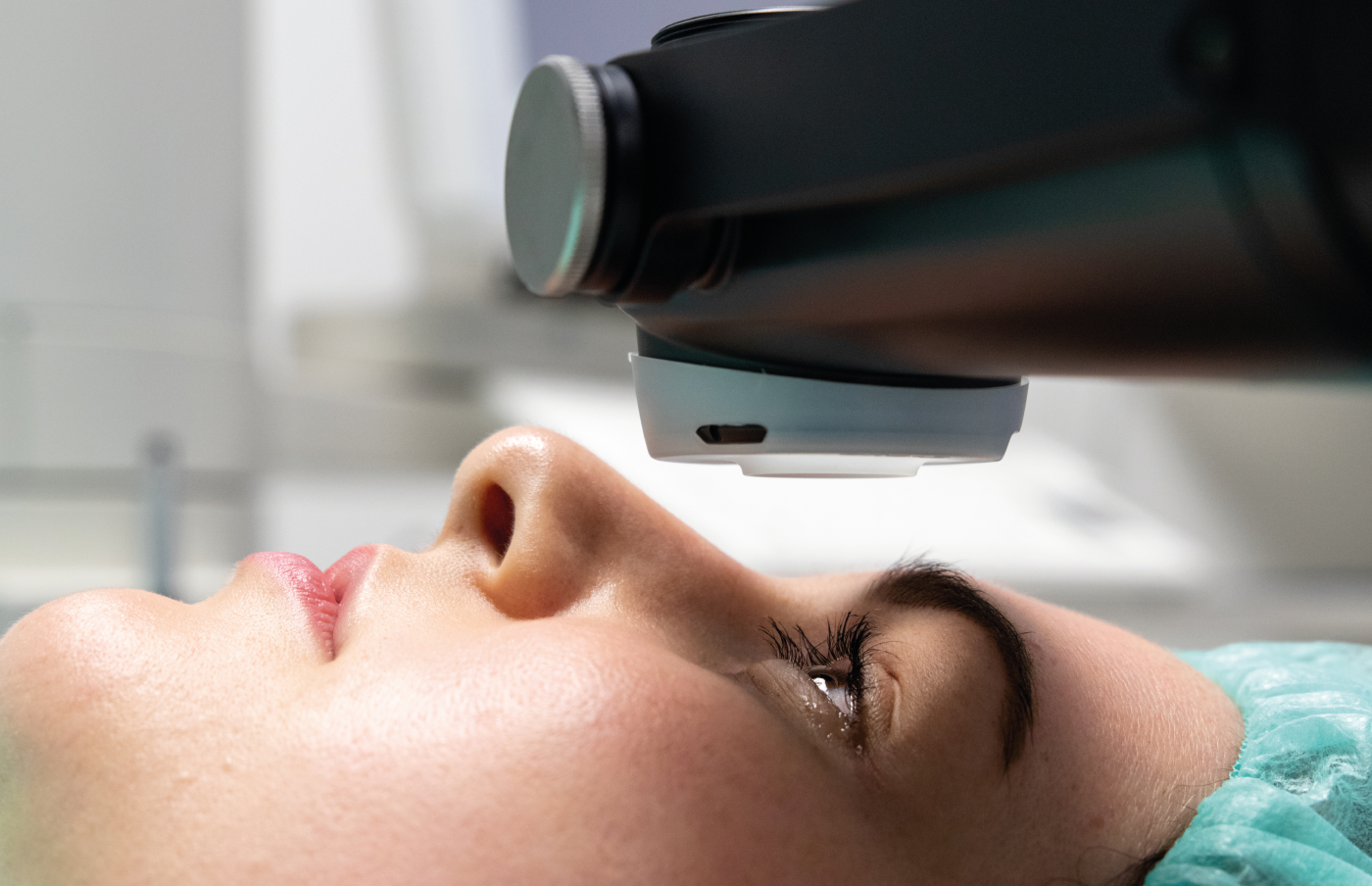
Before I move the story on from obtaining a CE mark – the European regulatory approval for our medical device – let me go back to a historical development that delayed our product’s arrival on the market. When I started my company, EMAGine, in 2012, I knew we needed to prepare the company to receive a CE mark on our UV lamp (now the C-Eye device, which allows corneal cross-linking at the slit lamp) and riboflavin (the procedure kit, our consumable product). We worked with an industrial designer for two years to prepare the initial simple concept plan, when suddenly, in 2014, the European Commission issued a statement disallowing the classification of riboflavin for ophthalmic use as a medical device. This left the industry in a state of panic, with the regulatory body no longer accepting dossiers to approve new ophthalmic solutions as medical devices. Many companies stopped supplying riboflavin solution once their CE marks expired and couldn’t be renewed. This meant a big delay for us; without riboflavin, our business model didn’t work. We decided to try our luck with manufacturers who had existing CE marks for riboflavin solutions and were able to secure one – not an easy feat.
After we received our CE mark in early 2020, we were hit by a completely unexpected disaster: COVID-19. We got regulatory approval right before the first lockdown started and we felt it was inappropriate to organize a big marketing and congratulatory campaign when the world was suffering so much. We decided to make a silent entry into the market, which allowed us to stay focused on our goals.

In our manufacturer we trust
We were very fortunate to partner with CSO Italia, a relatively small, but highly innovative company in Florence, Italy. The company was one of the designers of the Vega, one of the original cross-linking machines, which gave them a wealth of knowledge about optimizing a UV lamp. We decided to use their help not only with designing our device, but also with manufacturing it. Making the device ourselves would have meant a huge investment we were not prepared for, so it made sense to outsource it to a company with a lot of experience in cross-linking technology – and one we really trusted. CSO shares our belief in education, training, and ensuring clinical validation of our technology before it goes on to market. Obtaining such validation may delay market entry, but it also means that we know we can stand by the product we are promoting and selling.
Identifying target markets and distributors
We had to assess our key target markets in detail and decide whether we wanted to reach consumers directly or through distributors. The latter is a much better approach for a small startup company, so that’s what we chose. Of course, a customer might occasionally approach the company directly to buy a device, but if you really want to penetrate the market in a particular country and get a good share of it, you need people on the ground, living and breathing your product, representing you at national congresses and visiting clinicians’ offices. A good distributor can be your lifeline. We had to assess distributors in our specific niche market. Though we had the opportunity to use the existing networks of our leadership and CSO Italia’s, those were mostly among anterior segment specialists; we also needed distributors able to reach general ophthalmologists. In some countries, there’s just one successful distributor used by everyone in the field; in others, navigating distributor politics and competitor interests can be a difficult and complicated process.
“Selling” your product to distributors is very different to selling it to clinicians, with whom you can talk about research and applications. When you talk to distributors, what they really want to know is how to make the product successful in their market. You have to talk about unique selling points, capital recovery, competitor analysis… it’s a different conversation.
Negotiating distributor agreements is likely to give you sleepless nights. You have to be mindful of language and cultural differences and the fact that people do business in very different ways. We are currently dealing with 65 distributors representing 92 countries. All our business is conducted in English – and that is possibly the only common point we have. You have to take it point by point with each distributor, treat them equally (don’t pick favorites – the others will find out!), and keep track of all the arrangements. Good project management software is a must. Our manufacturer, CSO, has also helped us enormously in this process. Mutual trust between you and your distributors is crucial and it pays off.
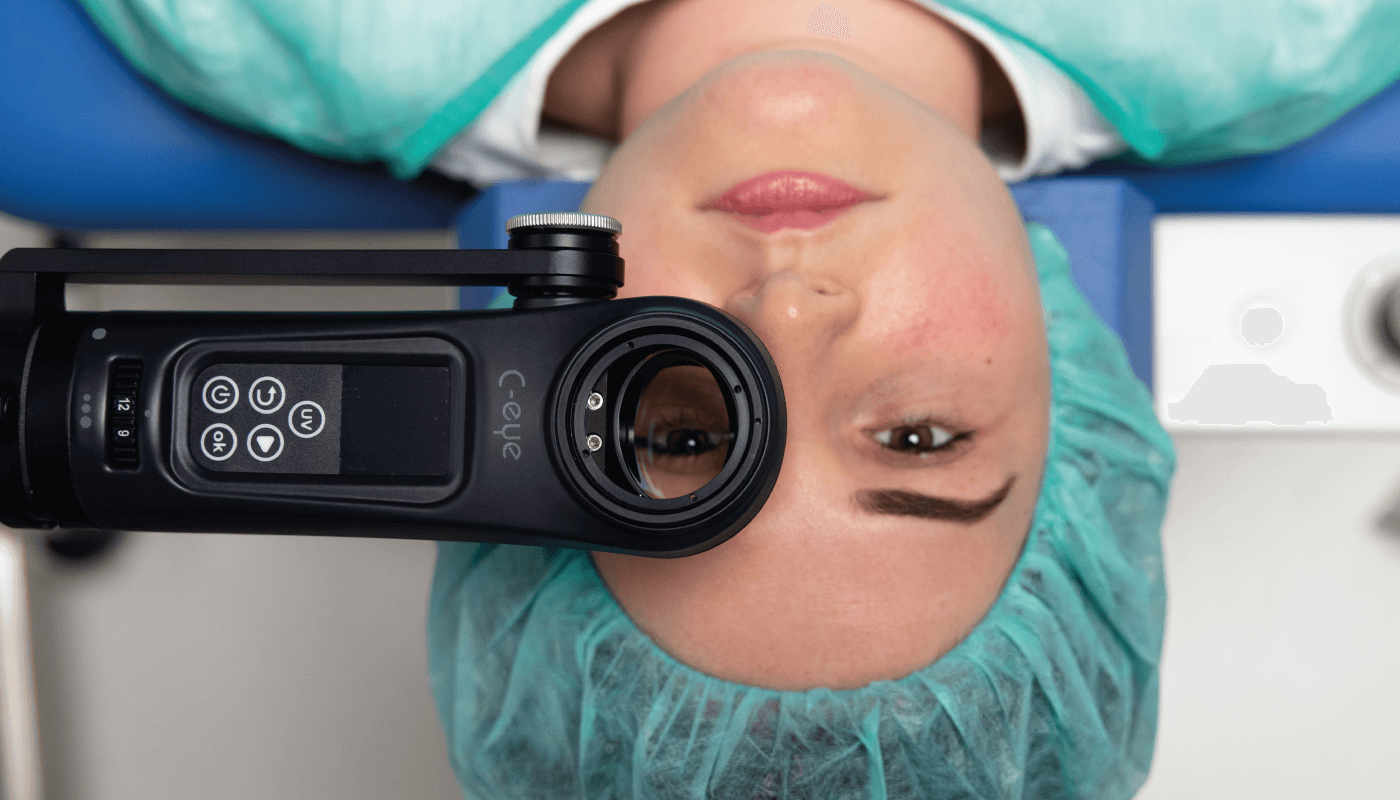
More regulatory affairs…
Once you have signed agreements, distributors need authorization to start selling the device in their markets. Each new market is like a new distributor; they all have different needs and requirements. A real win for us has been keeping all our standard documentation in an online, password-protected distributor portal that distributors can access at their convenience, only contacting us if they need additional information. We can update all documents to the latest versions instantly. We have also developed a distributor training video and uploaded it to our portal; distributors watch it before joining us for a live training webinar. We have collected all the questions distributors have asked and put them on the portal with answers – something they have found very useful when talking to customers. The feedback we’ve received has been amazing, and I feel that treating distributors like real partners and developing efficient processes has made obtaining authorizations much easier.
Lean, not mean
Early on, I decided to keep the company as lean as possible to allow us to make rapid business decisions. EMAGine has relied on four or five people assuming responsibility for all decisions, rather than creating the big chain of command larger companies with private equity might have. I wanted to be able to make quick technical changes and rapidly follow developments in the field. This strategy has paid off.
Though we may not have huge amounts of money to spend on promotion, we are dedicated to providing education and training. EMAGine has been lucky to have the support and backing of world-renowned medical and scientific experts and we have used this power, along with my own time and effort, to organize wet labs to show doctors how simple and portable our system is. We have attended national and international meetings to demonstrate the usefulness of the device. My husband, Farhad Hafezi, offers to provide additional educational courses at meetings he is invited to attend, and these have always been well-received. When COVID-19 struck, we organized countless virtual courses and webinars. Ophthalmologists from all over the world wanted to see what cross-linking at the slit lamp was about – and we were ready to demonstrate it to them. Our command of the (virtual) podium has served us well, allowing us to disseminate information via free educational webinars. We are always happy to provide free training and support to anyone interested in our device.
Don’t underestimate the power of social communication channels for promoting your product. Even if you are not a natural social media person, you have to engage with doctors and the industry, so make sure you use LinkedIn, Facebook, and Instagram. I never used Facebook until I noticed how much it was used for business in the Middle East and North Africa – in some places, it is a primary mode of communication! Within four months of setting up my Facebook profile, I went from zero to 4,000 contacts. This really helped me promote webinars, reaching people I wouldn’t have been able to reach otherwise.
Bridging the gap
I’m passionate about providing care to patients in remote areas in real need of our technology. This makes sense not only from an altruistic point of view, but also for our business model. The biggest costs for our users is the riboflavin – the consumable – which might be a barrier to ophthalmologists in low- and middle-income countries. I would like to subsidize those costs, which would increase usage, increase our production amounts, and lower our production costs, allowing us to decrease the cost of our products across the board, even at an increased profit margin.
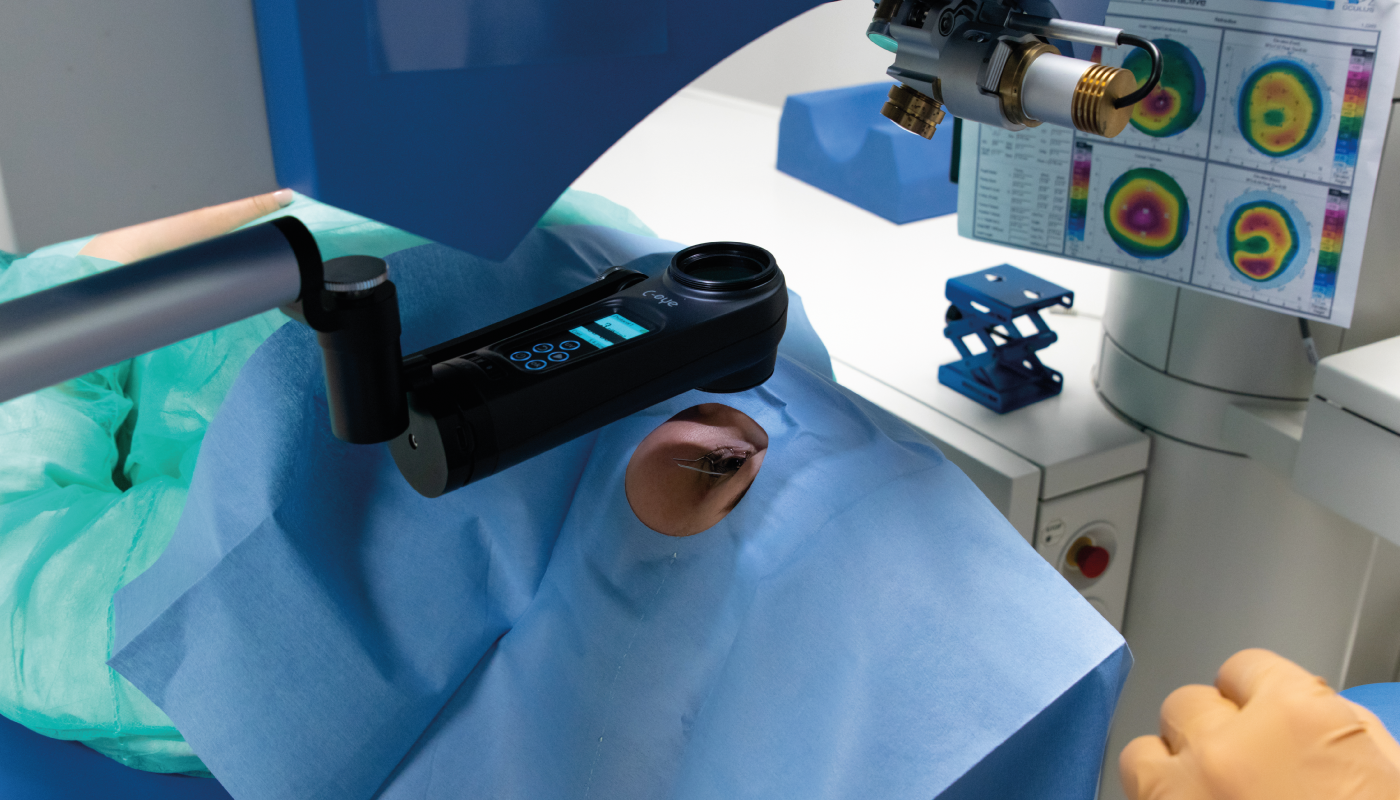
Using my experience as a fundraiser for nonprofit organizations, I’m calling on my network to present new concepts of treatment modalities in select countries, such as Yemen or Nepal, where there is a high incidence rate of corneal infections. We understand that funding agents are interested in sustainable programs, so we have to find partners on the ground who will make that possible – most likely NGOs. The plan for EMAGine is to provide products at cost and raise funds to offset the cost of consumables and shipping. We would, of course, provide all the education and training local ophthalmologists need.
We have a brilliant team, and a wonderful product that has the potential to change the field, and I want to make this technology available to the people who need it the most. Commercializing my product has not been easy, but it has been really rewarding – and great fun. And if anyone can use the lessons I have learned on my journey to ease their own, it will make my time and effort over the past few years even more worthwhile.