Crystallins are the collection of structural proteins found in the lens of the eye that help to focus light onto the retina. We know that over our lifetimes they can accumulate damage, losing their native structure and sticking together to form aggregates – one of several mechanisms that causes cataracts. But how exactly does this happen – and can it be stopped? Eugene Serebryany, a Post-Doctoral Fellow at the Department of Chemistry and Chemical Biology at Harvard University, USA, wants to find out.
In 2015, Serebryany and his Harvard-MIT team made the crucial discovery that wild-type (undamaged) crystallin promoted aggregation of mutant (damaged) versions – without itself aggregating (1). Chemical bonds between sulfur atoms within the protein (disulfide bonds) were found to play a role in aggregation (2). Most recently, the team found that crystallin protein molecules engaged in oxidation–reduction reactions with one another – disproving the long-held assumption that crystallins are inert (3).
We spoke to Serebryany to find out more about the role of crystallins in cataract formation.
What led you to study crystallin proteins?
The eye lens proteome is fascinating because it has evolved to minimize light scattering. That means it must resist aggregation because protein aggregates (clumps of many molecules of a protein) scatter visible light. Yet, the protein molecules in the core region of the lens are synthesized before birth and never replaced thereafter. These protein molecules are undergoing an aging process even before we are born, and they continue to accumulate various kinds of damage throughout life.
The biochemistry and biophysics of this highly concentrated solution of highly aged proteins, and how they have evolved to resist aggregation, has been of huge interest to many researchers over the years. One of them was my PhD adviser at MIT, Jonathan King, who first introduced me to these fascinating molecules. Our current work in the Shakhnovich group at Harvard builds directly on those earlier studies.
Of course, as fascinating and unique as crystallins are for their own sake, they also have important implications for public health. Their aggregation – when it does eventually occur – causes cataracts, which affect tens of millions of patients. Some people falsely assume that modern surgery has solved the problem of cataracts.
In fact, millions of patients around the world never benefit from the surgery, either because they can’t afford it, because they live in areas where it is not available, or because surgery is contraindicated for them. Moreover, the total cost of cataract surgeries performed worldwide already adds up to tens of billions of dollars per year, and as the global population ages, cataract prevalence will rise dramatically. A non-surgical therapy would be of great benefit, and that is the ultimate goal of our research.
What is the role of crystallins in causing cataracts?
Proteins from the crystallin family make up the lion’s share of all protein molecules in the cells of the eye lens. Since they are never replaced, at least in the lens core region, they accumulate damage over a lifetime. Eventually these crystallin protein molecules begin to lose their native structure (the normal 3D arrangement of atoms) and stick together to form aggregates.
Once the aggregates reach a size that is comparable to the wavelength of visible light, they begin to scatter light, resulting in less light reaching the retina and blurring of the resulting image. Because blue light has the shortest wavelengths, it gets scattered the most, so the colors we see also change, becoming yellower.
How has your work contributed to our understanding of lens crystallins?
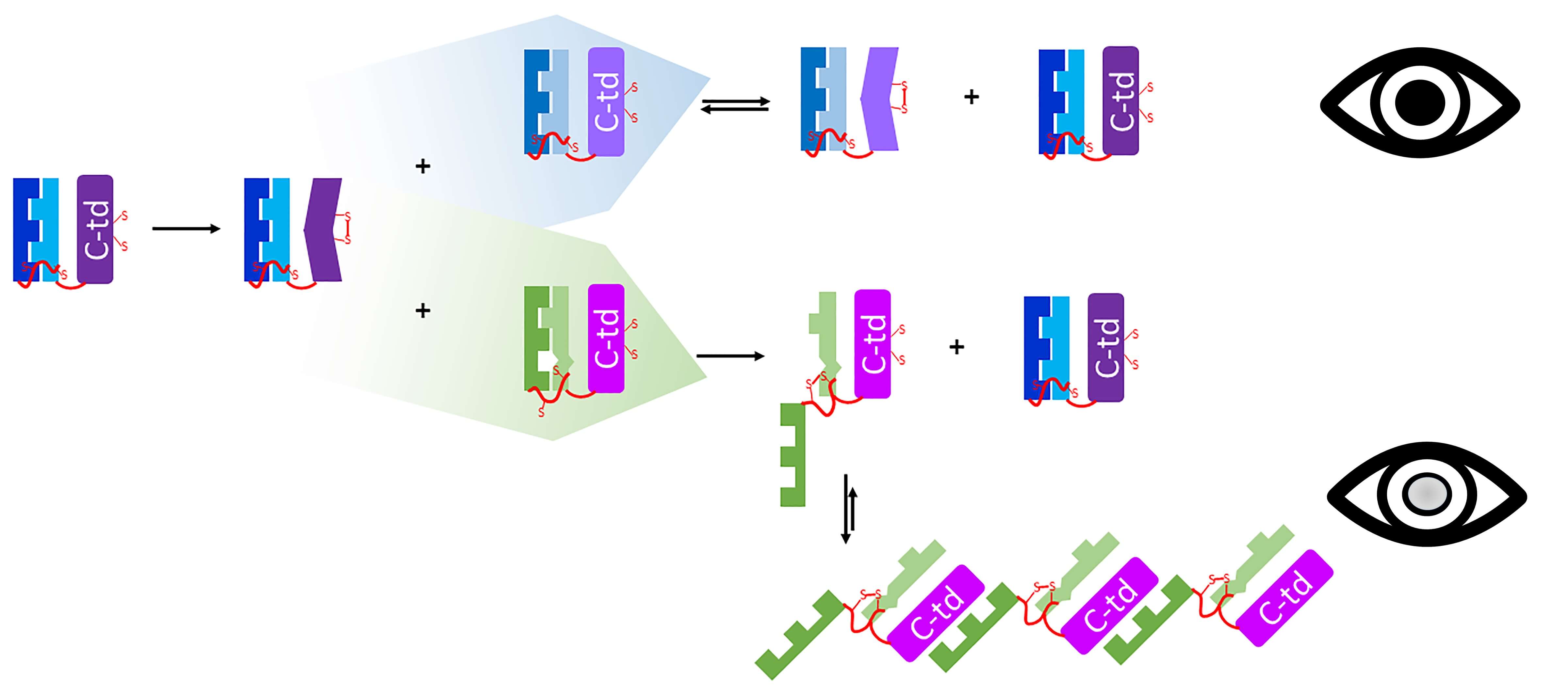
The initial observation that we reported in 2015 (1) was striking: mixing mutated protein with normal, unmutated protein led to rapid aggregation and a spike in light scattering. We were able to use gel electrophoresis, and more recently mass spectrometry, to separate the components of the aggregates and saw, to our surprise, that only the mutant protein was present there.
There are several health conditions elsewhere in the body in which misfolded mutant proteins cause otherwise normal (wild-type) proteins to misfold likewise – the mutant protein acts as a template. This is the mechanism behind prion diseases (such as Creutzfeldt-Jakob disease), and it leads to aggregation of both the mutant and the wild-type molecules. Our initial hypothesis was that a similar phenomenon could exist in eye lens crystallins. However, the truth turned out to be the reverse: a wild-type crystallin promoted aggregation of a mutant version of itself, without itself aggregating.
This new phenomenon was intriguing, but its mechanism remained totally mysterious. My collaborators and I have devoted the past several years trying to figure it out. The search for this mechanism led us to another surprise: a chemical reaction was taking place between these crystallin protein molecules, a process of oxidation–reduction (3). We also believe there is a second aggregation-promoting mechanism at work, which we are now studying.
You describe the chemical reaction as being like a “hot potato competition” – could you explain that?
We found that the crystallin proteins can pass disulfide bonds among themselves, from one molecule to another to another. These disulfide bonds are formed when two atoms of sulfur within one protein molecule react with each other. (This chemical reaction releases electrons, making it an oxidation reaction.)
The disulfide bond can then be transferred to another pair of sulfur atoms on a second molecule of this protein. In chemical terms, molecule 2 releases electrons that are received by molecule 1, so molecule 2 is oxidized and molecule 1 is reduced. These transfers of disulfides can be passed back and forth for a long time if the two molecules are equivalent.
How does that cause aggregation?
There are multiple reactive sulfur atoms in each molecule of gamma-crystallin. In wild-type protein, most of those sulfur atoms are hidden and therefore not available for this kind of chemical reaction. The situation changes if a mutation or another form of damage causes the protein’s structure to “loosen up”, exposing more sulfur atoms.
We previously showed (2) that if a pair of sulfur atoms that is normally hidden becomes exposed and forms a disulfide bond, the protein becomes trapped in an aberrant structure and becomes sticky, leading to aggregation. Studies by our colleagues in the Monnier and Fan groups at Case Western Reserve University strongly suggest that this type of chemistry underlies gamma-crystallin aggregation in the lenses of cataract patients.
Now we can connect the dots. Disulfide bonds are passed around among crystallin molecules like a hot potato: if they land on a damaged protein molecule that, due to its looser structure, displays sulfur atoms that it should have kept hidden, then this damaged molecule gets trapped in a sticky non-native structure, and forced to aggregate.
The disulfides do no great harm to the structurally sound crystallin molecules, and may even be protective, but they drive the structurally weakened molecules into aggregates that scatter light – hence, the aggregates in our experiment only contained mutant (damaged) proteins.
Can we stop proteins from aggregating?
The cells of the core region of the eye lens cannot make new protein molecules, nor can they actively degrade them. Peptide-based drugs would be expected to be rapidly broken down and metabolized in any other part of the body, but not in the nucleus of the lens. Although we haven’t yet reported any results with potential peptide drug candidates, we are pursuing several that we believe could inhibit aggregation by affecting both structure and chemistry.
We anticipate that the main challenge will not be finding peptides that can inhibit aggregation, but rather delivering such peptides to the most vulnerable cells of the lens. Peptide drugs tend to be large molecules, and we don’t yet know if they will penetrate the tissue in sufficient quantities. If not, all is not lost – the Arora group at New York University has already shown that it is possible to mimic peptide drugs with much smaller non-peptide ones, if necessary.
What do these findings mean for the future of cataract research?
They advance our understanding of the mechanisms behind what is likely the most common type of cataract (though there are other types with clearly distinct mechanisms). Still, there is much more work to be done.
Efforts to treat cataracts therapeutically have grown in number and made waves in recent years; at least two distinct lipid-based treatment approaches are now being pursued, for example. No drugs have been approved so far, and ultimately, a combination of drugs might be needed. But the more we learn about the biochemistry and biophysics of cataract formation, the wider the space of therapeutic possibilities will be.

