- Genetic testing can benefit children with a range of eye conditions, including Bilateral childhood cataract, congenital glaucoma, albinism and retinal dystrophy
- Genetic testing has the potential to provide a precise diagnosis, guide management, help guide prognosis and provide information to other family members
- New developments in sequencing techniques allow for fast and relatively inexpensive genetic testing
- In the future, genetic testing will be offered to all families suspected of having a genetic condition.
We now understand much more about the genetic basis of many eye diseases, including those relevant to children – for example, inherited retinal disease, and childhood forms of glaucoma, cataract and albinism. In parallel, we have seen a transformation in genetic testing technology; it is faster, cheaper and more readily available than ever before. These developments are changing the way we manage ocular diseases in children: increasingly, it is standard practice to integrate genetic tests into the management of these conditions.
Faster, better, cheaper
Key developments in this ongoing evolution include the advent of fast, sophisticated sequencing capabilities: specific options include microarray screening; single gene tests; Next Generation Sequencing panels (which allow parallel sequencing of multiple genes), whole genome sequencing. Today, we can analyze several hundred relevant genes at once, or rapidly sequence the entire genome – or exome – and then focus on the most relevant genes.
Thus, the days when genetic tests were slow, inconsistent and patchily accessible are long gone. Furthermore, while speed and efficiency have increased, costs have dramatically reduced: we can now sequence a panel of genes for about £800 (~$1000). The UK’s Hundred Thousand Genome Project, initiated by the Conservative government in 2012, aimed to sequence the genomes of 100,000 NHS patients with rare diseases or cancer. The idea was to generate clinically relevant data that will assist disease management, and would almost certainly result in the development of new genetic tests.
These developments in turn have triggered organizational change. Last year, Genomics England reorganized NHS genetic services, such that genetic tests are now centrally funded and will be accessible to everyone in the UK – regardless of postcode. All patients now have access to genetic testing– there is no longer any inequality arising as a result of geographical location.
Greater impact
There’s no doubt of the power of the new technology – but what clinical impact does it have? Many pediatric eye disorders may benefit from genetic testing (see Childhood eye disorders that benefit from genetic testing).
- Bilateral cataracts
- Bilateral congenital or developmental glaucoma
- Retinal dystrophy
- Albinism
- Bilateral anterior segment dysgenesis
- Foveal hypoplasia
- Lens abnormalities such as ectopia lentis
- Corneal dystrophy
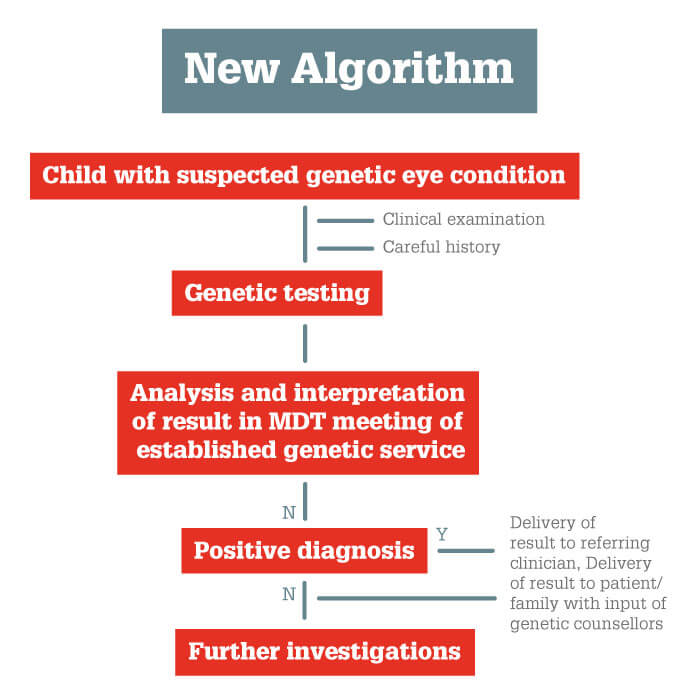
Our work shows that in about 70 percent of patients with childhood cataracts, genetic screening provides a diagnosis that guides clinical management (1, 2, 3). That’s why we now offer children with bilateral cataracts genetic testing at an early stage of the care pathway (Figure 1): this new algorithm avoids the need for lengthier, less reliable investigations, by providing an accurate diagnosis much more quickly.
Real examples
In many pediatric ocular conditions, identification of the underlying genetic cause is critical for optimal disease management – some of the causative syndromes can affect prognosis and development, and therefore need targeted treatment. For example, children with Cockayne’s syndrome may first be referred due to cataracts and deep-set eyes, but due to their systemic issues they will have a severely restricted lifespan. Patients with oculocardiodental syndrome for example need screening for cardiac issues.
Bilateral childhood cataract is a good example of a pediatric ocular condition that benefits from the new genetic tests (see Box above). It is not, in itself, a diagnosis, as congenital cataracts have variable systemic causes, reflected in very variable phenotypes (Figure 2). In particular, bilateral cataracts may arise as a consequence of metabolic deficiencies, such as galactosaemia, galactokinase deficiency and cerebrotendinous xanthomatosis. Therefore, this condition is often the first sign of a serious underlying disorder that can and should be effectively treated. Of course, this presumes an accurate and early diagnosis – all too rare in the past, when children with bilateral cataract were referred to pediatricians for extensive, time-consuming investigations that rarely revealed the actual cause. Today, however, interrogation of the NGS panel provides far better information in far less time. Indeed, we can point to a number of cases from our clinic in which the new technology has significantly guided clinical decisions, to the great benefit of patients and their families (see Boxes).
Situation: After failing a school-based vision screen, a five-year old child was found to have bilateral posterior cataracts that required surgery (lensectomies, IOLs). At age 11, he was found to have learning disabilities (IQ of 64, autistic spectrum).
Actions: We ran a genetic test using an NGS cataract panel that can identify heterozygous mutations in sterol-C5-desaturase (SC5D). This suggested a diagnosis of lathosterolosis, an extremely rare congenital disorder that affects sterol biosynthesis. The diagnosis was confirmed by blood tests indicating raised plasma lathosterol (219 mmol/l, as compared to the normal range of 0.53 -16 mmol/l).
Outcome: The correct diagnosis enabled the patient to be prescribed the correct treatment (simvastatin), which is thought to prolong life and arrest further neurological damage. Diagnosis of this disorder, where the parents will have a 25 percent chance of having an affected child with each pregnancy, also allows appropriate counseling of the patient’s family; for example, with regard to prenatal diagnosis options (5).
Genetic tests are also very helpful for guiding treatment of children with retinal dystrophy, not least because there are several hundred variants of this condition. Some variants are stable, others progress to blindness; and some are associated with systemic effects that must be specifically managed. Note too that a small number of retinal dystrophies are the subject of clinical trials (for example, gene therapy for RPGPR X-linked retinitis pigmentosa: NightStar Therapeutics) or novel treatment options (such as RPE65 Leber congenital amarosis gene therapy: Spark Therapeutics). Clearly, knowledge of the exact basis of retinal dystrophy is critical for accurate prognosis and appropriate management of these children. Our approach therefore has been to test with an NGS retinal panel that includes genes associated with systemic conditions, such as Bardet-Biedl and Senior-Loken syndromes. We have found that this approach provides a diagnosis for 80 percent of children with retinal dystrophy (4). Having an exact diagnosis in these patients really can be very beneficial; for example, we may be able to identify that the patient has a stationary rather than progressive retinal dystrophy; obviously, it’s a relief for the parents to know that the vision is unlikely to deteriorate.
Cases of bilateral childhood glaucoma (see second Box below) may also benefit from genetic tests, as there is usually a genetic cause. And again, the condition may be associated with other ocular or systemic effects. A precise diagnosis can be enormously helpful for the patient’s family, as you can reassure them that there is no other condition to worry about, for example, or warn them of the risk of subsequent babies being similarly affected.
Children with albinism may be offered genetic testing; most of the many different types of albinism affect only skin, hair and eyes, but some forms are associated with systemic problems, such as Hermansky-Pudlak syndrome (compromised erythrocytes, bleeding problems) and Chédiak-Higashi syndrome (compromised leucocytes, recurrent infections). Both of these conditions can be treated once diagnosed – but, without genetic tests, they may not be diagnosed for many years. Genetic tests can also be helpful in equivocal albinism cases; for example, providing an early diagnosis of X-linked ocular albinism. Thus, the technology can provide a firm diagnosis far earlier than is possible with non-genetic tests.
Situation: A baby presented with severe bilateral congenital glaucoma, lamellar cataract right eye; underwent penetrating keratoplasty for corneal opacity. History of intra-uterine growth retardation and failure to thrive. Consanguineous parents.
Actions: Genetic test with an NGS panel revealed presence of CYP1B1 homozygous mutation, which is known to be associated with congenital glaucoma.
Outcome: Diagnosis enabled us to inform parents that this is an isolated condition, such that the poor growth and delayed development are not due to a broader metabolic syndrome (more likely due to general anesthetics and steroid treatments). Also enabled parents to be counseled regarding the risk of subsequent babies being similarly affected.
There are limits…
Nevertheless, genetic testing is not appropriate in all circumstances; in particular, it is not required when the condition is completely unilateral, unless there is some systemic association or known family history. Similarly, it’s not necessary if we already have a genetic diagnosis within the family, unless we need to confirm the condition is also present in the patient. Equally, some patients simply don’t want to have a genetic test, and in others the test result will make no difference to their management.
We should also remember that, even today, genetic tests are not always perfect, and occasionally raise problematic issues. In some cases, they identify mutations that are of are of unknown significance, as they have not been described before or it is unknown whether they would in fact have a detrimental effect. In other cases, they indicate that the patient is a carrier of a different condition, which gives the problem of whether and how to inform them. And sometimes the test has implications for systemic health and/or for future life decisions – including the option of prenatal diagnosis in subsequent pregnancies. We must manage these issues as diligently as we can: test results should be discussed in a multidisciplinary team meeting before information is issued to the referring clinician; and the patient is informed of the test result via an established genetic service, with counseling as necessary.
Information is not knowledge
In summary, I believe genetic testing will increasingly become a fundamental component of pediatric ophthalmology – and indeed of clinical ophthalmology as a whole. Eventually, whole genome sequencing will be offered to all families suspected to have a genetic condition. It will remain the case, however, that expert interpretation of the test result shall be of critical importance; equally, careful delivery of this qualified information to the patient, together with access to appropriate family counseling, will always be paramount.
Looking further ahead, there have been suggestions that genetic tests could be made available to everybody, not just those suspected of having a genetic disease in their family. In my opinion, we don’t yet know enough about the implications of particular genotypes with regard to lifetime health, and providing raw information without knowledgeable interpretation may just worry people for no good reason.
Let’s just focus on the people who really need these tests: those with congenital ocular conditions who are without a precise diagnosis, and those who are carriers and considering having children. In these patients, it is clear that today’s genetic tests provide faster and more precise diagnoses, which in turn allow increasingly accurate prognoses, better counseling, and more timely, exactly-targeted treatment. The next generation is fortunate indeed!
References
- RL Gillespie et al., “Personalized diagnosis and management of congenital cataract by next-generation sequencing”, Ophthalmology, 121, 2124 (2014). PMID: 25148791. RL Gillespie et al., “Next-generation sequencing in the diagnosis of metabolic disease marked by paediatric cataract”, Ophthalmology, 123, 21 (2016). PMID: 2623629. M Musleh et al., “Diagnosing the cause of bilateral paediatric cataracts: comparison of standard testing with a next-generation sequencing approach”, Eye, 30, 1175 (2016). PMID: 27315345. RL Taylor et al., “Panel-based clinical genetic testing in 85 children with inherited retinal disease”, Ophthalmology, 124, 985 (2017). PMID: 28341476. R Anderson et al., “Lathosterolosis: A relatively mild case with cataracts and learning difficulties”, JIMD Rep, 44, 79 (2019). PMID: 30097991.
