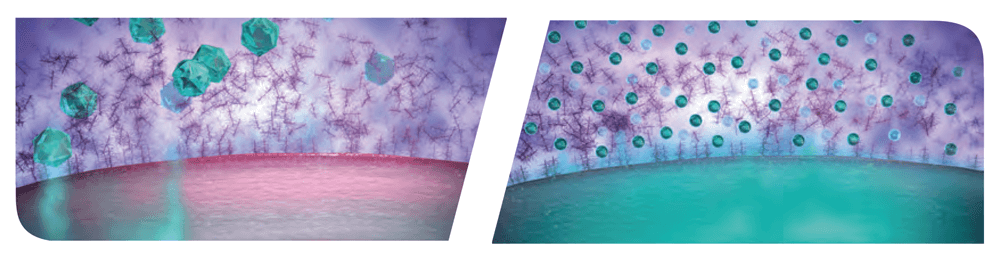
 Figure 1. Comparison of traditional corticosteroid (left) with AMPPLIFY® (right), which is designed to enhance ocular surface tissue distribution and penetration to the sites of Dry Eye-related ocular surface inflammation, specifically the cornea and conjunctiva (3, 5).
Figure 1. Comparison of traditional corticosteroid (left) with AMPPLIFY® (right), which is designed to enhance ocular surface tissue distribution and penetration to the sites of Dry Eye-related ocular surface inflammation, specifically the cornea and conjunctiva (3, 5).
Kala Pharmaceuticals is focused on the discovery, development, and commercialization of innovative therapies for eye disease. The biopharmaceutical company’s two marketed products, EYSUVIS® (loteprednol etabonate ophthalmic suspension) 0.25% and INVELTYS® (loteprednol etabonate ophthalmic suspension) 1.0%, both use its proprietary AMPPLIFY® drug-delivery technology, designed to enhance drug distribution and penetration to the ocular surface.
While mucus plays a central role in protecting the body from harmful foreign materials, it hinders the ability of medications to penetrate mucus-protected tissues, thereby potentially reducing therapeutic effect (1, 2). Kala’s proprietary AMPPLIFY® mucus penetrating particle (MPP) drug-delivery technology is designed to enhance distribution and penetration through the mucus barrier and deliver increased concentration of drug to the target ocular surface tissues, specifically the cornea and conjunctiva (3, 5).
“Our AMPPLIFY® technology is what enabled our first two products,” says President & Chief Operating Officer, Todd Bazemore. “It has allowed us to help meet the need within the ophthalmic community for effective drug delivery to the ocular surface. EYSUVIS and INVELTYS have already had a significant impact on the ability of eye care professionals to effectively and efficiently treat their appropriate patients.”
The Beginning
The MPP technology originated out of the largest biomedical engineering lab in the world, run by Robert S. Langer at the Massachusetts Institute of Technology (MIT). Langer is a world-renowned researcher in biotechnology, especially in the fields of drug delivery systems and tissue engineering. AMPPLIFY® uses nanoparticles (~300 nm in diameter) engineered via surface modification to penetrate through mucus pores (approximately 500 nm in diameter) to the ocular surface without being bound up and eliminated by the tear film (1, 2, 3, 4, 5). In a preclinical study, AMPPLIFY® technology increased loteprednol etabonate penetration to the cornea and into the aqueous humor by more than three times compared with loteprednol etabonate without AMPPLIFY® (5).
EYSUVIS: The only short-term Rx treatment in dry eye disease (DED)
In October 2020, EYSUVIS (loteprednol etabonate ophthalmic suspension) 0.25% became the first and only FDA-approved prescription corticosteroid therapy for the short-term (up to two weeks) treatment of the signs and symptoms of dry eye disease, including dry eye flares.
As with other ophthalmic corticosteroids, EYSUVIS is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella. Additionally, EYSUVIS should not be used in the presence of a mycobacterial infection of the eye and fungal diseases of ocular structures.
Since DED has always been treated as a chronic condition, diagnosing and treating patients with dry eye flares is transforming the treatment paradigm. Research states that 75–90 percent of dry eye patients report suffering from acute episodes of worsening symptoms (dry eye flares) lasting a few days to a couple of weeks, even if they are currently being treated with OTC or chronic dry eye medications (6, 7, 8, 9). EYSUVIS may be an appropriate acute therapy for dry eye patients to treat these flares. Eye care professionals can extinguish present flares and also provide symptomatic relief of future flares by ensuring appropriate patients have EYSUVIS on hand for those times throughout the year.
“EYSUVIS has the potential to be complementary to existing therapies and offers a differentiated product profile for the short-term treatment of DED, including the management of dry eye flares,” Bazemore says. “Kala has received extensive positive feedback from eye care professionals on the rapid onset of effect and safety profile of EYSUVIS. It fills an unmet need as the only FDA approved short-term therapy to treat signs and symptoms of dry eye disease, including dry eye flares,” (4).
Furthermore, EYSUVIS demonstrated a well-tolerated safety profile in clinical trials. Focusing on IOP data, the treatment and vehicle groups, respectively, found 0.2 percent and 0.0 percent of subjects experienced a ≥10 mm Hg increase from baseline resulting in an intraocular pressure measurement of ≥21 mm Hg at any post-baseline visit up to 29 days (10). Please see Important Safety Information below.
Collaboration with eye care
Since its founding in 2009, Kala has focused on innovation and its relationship with eye care professionals. The company is dedicated to understanding and meeting their needs, as well as those of their patients, thereby maximizing its overall impact in the ophthalmic space.
“Kala is a company that was formed with an emphasis on listening to the eyecare community,” says Bazemore. “From day one, we’ve listened to leading eye care professionals to understand how new products could benefit practices and more importantly patients.”
As Kala prepared to bring EYSUVIS to market, the company discovered that there was a disconnect between the number of patients reporting acute worsening of symptoms – or dry eye flares – and the number of patients eye care professionals reported as experiencing flares. “We began to understand that, historically, there hasn’t been a lot of proactive discussion about dry eye flares, because there wasn’t an FDA-approved Rx product for the short-term treatment of dry eye disease,” Bazemore comments. “Now there is, and the discussion between eye care professionals and their patients can change.”
Future action
As the prevalence of dry eye disease continues to rise due to both the aging of the population as well as the ever-increasing amount of screen time, ocular surface health will remain a public health concern. EYSUVIS is poised to play a pivotal and growing role in the treatment arsenal. “We plan to be a leader in ophthalmic innovation with products targeted to treat anterior and posterior segment diseases, helping doctors to care more completely for their patients,” says Bazemore.
IMPORTANT SAFETY INFORMATION
Contraindication:
EYSUVIS, as with other ophthalmic corticosteroids, is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures.
Warnings and Precautions:
Delayed Healing and Corneal Perforation: Topical corticosteroids have been known to delay healing and cause corneal and scleral thinning. Use of topical corticosteroids in the presence of thin corneal or scleral tissue may lead to perforation. The initial prescription and each renewal of the medication order should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and, where appropriate, fluorescein staining.
Intraocular Pressure (IOP) Increase:
Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, as well as defects in visual acuity and fields of vision. Corticosteroids should be used with caution in the presence of glaucoma. Renewal of the medication order should be made by a physician only after examination of the patient and evaluation of the IOP
Cataracts:
Use of corticosteroids may result in posterior subcapsular cataract formation.
Bacterial Infections: Use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, corticosteroids may mask infection or enhance existing infection.
Viral Infections:
Use of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution. Use of ocular corticosteroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex).
Fungal Infections:
Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local corticosteroid application. Fungus invasion must be considered in any persistent corneal ulceration where a corticosteroid has been used or is in use.
Adverse Reactions:
The most common adverse drug reaction following the use of EYSUVIS for two weeks was instillation site pain, which was reported in 5% of patients.
INDICATION
EYSUVIS is a corticosteroid indicated for the short-term (up to two weeks) treatment of the signs and symptoms of dry eye disease.
View full Prescribing Information (https://www.eysuvis.com/pdf/ prescribing-information.pdf ).
US--2100042
EYSUVIS (loteprednol etabonate ophthalmic suspension) 0.25%, for topical ophthalmic use
BRIEF SUMMARY OF FULL PRESCRIBING INFORMATION
INDICATIONS AND USAGE
EYSUVIS is a corticosteroid indicated for the short-term (up to two weeks) treatment of the signs and symptoms of dry eye disease.
CONTRAINDICATIONS
EYSUVIS, as with other ophthalmic corticosteroids, is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures.
WARNINGS AND PRECAUTIONS
Delayed Healing and Corneal Perforation—Topical corticosteroids have been known to delay healing and cause corneal and scleral thinning. Use of topical corticosteroids in the presence of thin corneal or scleral tissue may lead to perforation. The initial prescription and each renewal of the medication order should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and, where appropriate, fluorescein staining.
Intraocular Pressure (IOP) Increase—Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, as well as defects in visual acuity and fields of vision. Corticosteroids should be used with caution in the presence of glaucoma. Renewal of the medication order should be made by a physician only after examination of the patient and evaluation of the IOP.
Cataracts—Use of corticosteroids may result in posterior subcapsular cataract formation.
Bacterial Infections—Use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions of the eye, corticosteroids may mask infection or enhance existing infection.
Viral Infections—Use of corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution. Use of ocular corticosteroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex).
Fungal Infections—Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local corticosteroid application. Fungus invasion must be considered in any persistent corneal ulceration where a corticosteroid has been used or is in use. Fungal cultures should be taken when appropriate.
Risk of Contamination—Do not to allow the dropper tip to touch any surface, as this may contaminate the suspension.
Contact Lens Wear—The preservative in EYSUVIS may be absorbed by soft contact lenses. Contact lenses should be removed prior to instillation of EYSUVIS and may be reinserted 15 minutes following administration.
ADVERSE REACTIONS
Adverse reactions associated with ophthalmic corticosteroids include elevated intraocular pressure, which may be associated with infrequent optic nerve damage, visual acuity and field defects, posterior subcapsular cataract formation, delayed wound healing and secondary ocular infection from pathogens including herpes simplex, and perforation of the globe where there is thinning of the cornea or sclera.
Clinical Trials Experience—Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The most common adverse reaction observed in clinical trials with EYSUVIS was instillation site pain, which was reported in 5% of patients.
USE IN SPECIFIC POPULATIONS
Pregnancy—Risk Summary: There are no adequate and well controlled studies with loteprednol etabonate in pregnant women. Loteprednol etabonate produced teratogenicity at clinically relevant doses in the rabbit and rat when administered orally during pregnancy. Loteprednol etabonate produced malformations when administered orally to pregnant rabbits at doses 1.4 times the recommended human ophthalmic dose (RHOD) and to pregnant rats at doses 34 times the RHOD. In pregnant rats receiving oral doses of loteprednol etabonate during the period equivalent to the last trimester of pregnancy through lactation in humans, survival of offspring was reduced at doses 3.4 times the RHOD. Maternal toxicity was observed in rats at doses 347 times the RHOD, and a maternal no observed adverse effect level (NOAEL) was established at 34 times the RHOD. The background risk in the U.S. general population of major birth defects is 2 to 4%, and of miscarriage is 15 to 20%, of clinically recognized pregnancies. Data—Animal Data: Embryofetal studies were conducted in pregnant rabbits administered loteprednol etabonate by oral gavage on gestation days 6 to 18, to target the period of organogenesis. Loteprednol etabonate produced fetal malformations at 0.1 mg/kg (1.4 times the recommended human ophthalmic dose (RHOD) based on body surface area, assuming 100% absorption). Spina bifida (including meningocele) was observed at 0.1 mg/kg, and exencephaly and craniofacial malformations were observed at 0.4 mg/kg (5.6 times the RHOD). At 3 mg/kg (41 times the RHOD), loteprednol etabonate was associated with increased incidences of abnormal left common carotid artery, limb flexures, umbilical hernia, scoliosis, and delayed ossification. Abortion and embryofetal lethality (resorption) occurred at 6 mg/kg (83 times the RHOD). A NOAEL for developmental toxicity was not established in this study. The NOAEL for maternal toxicity in rabbits was 3 mg/kg/day. Embryofetal studies were conducted in pregnant rats administered loteprednol etabonate by oral gavage on gestation days 6 to 15, to target the period of organogenesis. Loteprednol etabonate produced fetal malformations, including absent innominate artery at 5 mg/kg (34 times the RHOD); and cleft palate, agnathia, cardiovascular defects, umbilical hernia, decreased fetal body weight and decreased skeletal ossification at 50 mg/kg (347 times the RHOD). Embryofetal lethality (resorption) was observed at 100 mg/kg (695 times the RHOD). The NOAEL for developmental toxicity in rats was 0.5 mg/kg (3.4 times the RHOD). Loteprednol etabonate was maternally toxic (reduced body weight gain) at 50 mg/kg/day. The NOAEL for maternal toxicity was 5 mg/kg. A peri-/postnatal study was conducted in rats administered loteprednol etabonate by oral gavage from gestation day 15 (start of fetal period) to postnatal day 21 (the end of lactation period). At 0.5 mg/kg (3.4 times the clinical dose), reduced survival was observed in live-born offspring. Doses ≥ 5 mg/kg (34 times the RHOD) caused umbilical hernia/incomplete gastrointestinal tract. Doses ≥ 50 mg/kg (347 times the RHOD) produced maternal toxicity (reduced body weight gain, death), decreased number of live-born offspring, decreased birth weight, and delays in postnatal development. A developmental NOAEL was not established in this study. The NOAEL for maternal toxicity was 5 mg/kg.
Lactation—There are no data on the presence of loteprednol etabonate in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for EYSUVIS and any potential adverse effects on the breastfed infant from EYSUVIS.
Pediatric Use—Safety and effectiveness in pediatric patients have not been established.
Geriatric Use—No overall differences in safety and effectiveness have been observed between elderly and younger adult patients.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility—Long-term animal studies have not been conducted to evaluate the carcinogenic potential of loteprednol etabonate. Loteprednol etabonate was not genotoxic in vitro in the Ames test, the mouse lymphoma thymidine kinase (tk) assay, in a chromosome aberration test in human lymphocytes, or in vivo in the single dose mouse micronucleus assay. Treatment of male and female rats with 25 mg/kg/day of loteprednol etabonate (174 times the RHOD based on body surface area, assuming 100% absorption) prior to and during mating caused pre-implantation loss and decreased the number of live fetuses/live births. The NOAEL for fertility in rats was 5 mg/kg/day (34 times the RHOD).
For a copy of the Full Prescribing Information, please visit www.EYSUVIS.com.
Manufactured for:
Kala Pharmaceuticals, Inc.
Watertown, MA 02472
Part # 2026R02
Marks designated by TM or ® are owned by Kala Pharmaceuticals, Inc. Patented. www.kalarx.com/patents © 2020 Kala Pharmaceuticals, Inc. All rights reserved. October 2020
Kala®
US-EYS-2000115
References
- SS Olmsted et al., Biophys J. 81, 1930 (2001). PMID: 11566767.
- HH Sigurdsson et al., “Int J Pharm, 453, 56 (2013). PMID: 23727593.
- PA Popov, J Ocul Pharmacol Ther, 36, 366 (2020). PMID: 32667250.
- Data on file. Kala Pharmaceuticals: Watertown, MA, USA.
- L Schopf et al., Ophthalmol Ther, 3, 63 (2014). PMID: 25134493.
- Based on a survey of 297 patients commissioned by Kala and performed by a third party.
- Based on a survey of 500 patients diagnosed with dry eye disease commissioned by Kala and performed by a third party.
- Based on a survey of 774 patients performed by a third party.
- Based on in-depth interviews with 30 patients performed by a third party.
- M Korenfeld et al., Cornea, 40, 564 (2021). PMID: 32826644.
