
Dry Eye Disease (DED) is one of the most common ocular surface disorders – and Meibomian Gland Dysfunction (MGD) is one of its most common causes. MGD leads to an unstable lipid layer and tear film evaporation from the eye. Standard treatment options for DED include lipid-containing artificial tears and thermomechanical relief of the eyelids. But now a novel investigational new drug aims to treat evaporative DED by addressing the lipid layer instability. NOV03 (100 percent perfluorohexyloctane) is a preservative-free ophthalmic solution with unique properties and the first drug in development that targets DED associated with Meibomian Gland Dysfunction – but what advantages does it have over traditional aqueous eye drops usually prescribed or recommended for DED?
Simply better...
Firstly, NOV03 is water-free, which means that i) microbial growth is not possible, therefore ii) the product requires no preservatives, and iii) the product has no pH and consequently does not effect native tear osmolarity. The implicit suggestion that NOV03 should be better tolerated than standard DED products is supported by preclinical studies: repeated up to 4 times daily instillation in rabbit eyes caused no irritation (1).
Secondly, the low surface tension of NOV03, an EyeSol®-based formulation, favors very effective spreading: an aqueous saline droplet “sits” on the cornea (contact angle: ~40 degrees), while EyeSol® droplets form a thin film on the tear film (contact angle: ~zero degrees) (1). Indeed, although EyeSol® droplet volumes are one-third those of saline, they cover a significantly greater corneal and eye surface area (1).
Thirdly, being amphiphilic, EyeSol® dissolves lipids and rapidly forms films on surfaces. Hence, NOV03 directly stabilizes the tear film without added surfactants, and has the ability to dissolve lipid deposits in blocked Meibomian glands (3). Preclinical studies show that topical EyeSol® formulations are sequestered in the tear film, where high levels persist for over 4 hours and penetrate into Meibomian glands, where significantlevels persist for up to 8 hours (2). Thus, the corneal coverage provided by NOV03 is not only rapid, but also durable.
A difference patients perceive
The above preclinical data are complemented by an increasingly large body of clinical evidence. We now know that perfluorohexyloctane administration improves MGD-associated DED by extending tear film break-up time, reducing corneal and conjunctival damage, increasing the number of expressible Meibomian glands, and improving Ocular Surface Disease Index (OSDI) values (4). Similarly, in patients with mild to moderate DED, NOV03 (four times daily) mediated >6 percent increase in tear film thickness and >13 percent increase in lipid layer thickness (5). And now a large US Phase II trial (below) further adds to the case for NOV03.
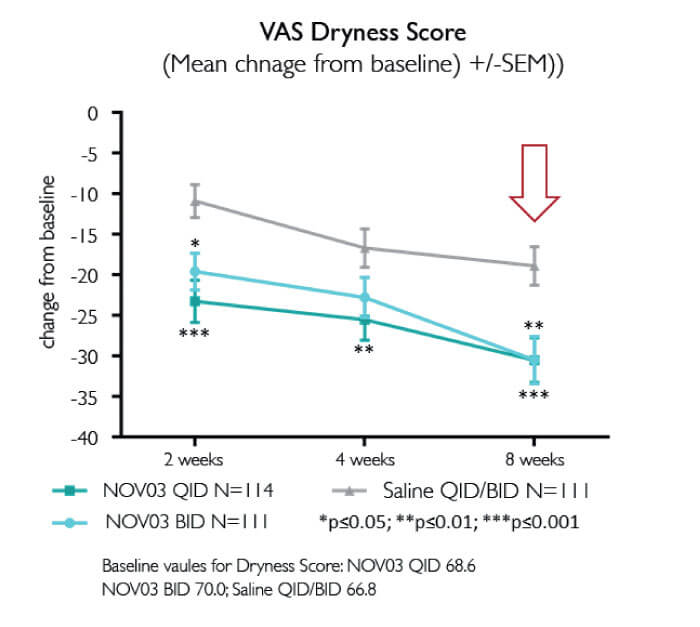
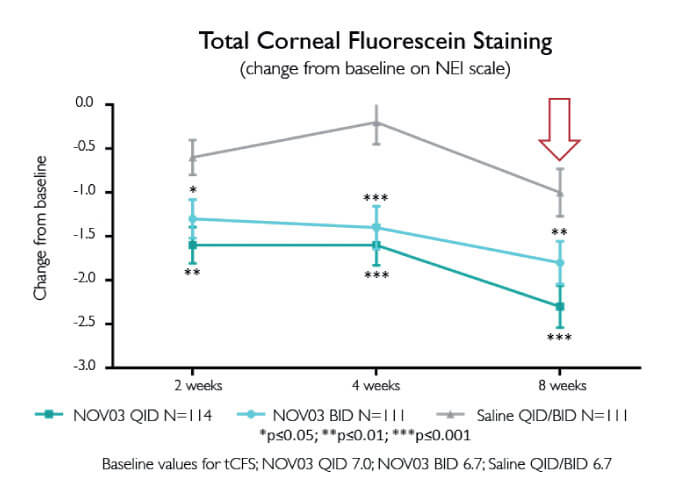
The SEECASE results are clear (6): excellent safety and tolerability, and outstanding efficacy (Figure 1, 2). Significant symptomatic improvements were apparent at two weeks and persisted until study completion; given that the study included only patients with relatively severe DED symptoms, this result is extremely encouraging. Also of note are the dose dependent outcomes observed in SEECASE: the four times daily regime gives better outcomes than the two times daily regime (Figure 1, 2).
What I Saw in SEECASE
Joseph Tauber’s referral-based dry eye center in Kansas City welcomes both DED and MGD patients. To date, he has participated in over 130 multi-center trials as principal investigator – including the SEECASE study. Here, he shares his thoughts with The Ophthalmologist.
MGD lacks both diagnostic and treatment tools; hence, therapy largely relies on home eyelid hygiene, although oral agents may improve Meibomian secretions in some patients. Similarly, DED treatments are poorly efficacious, slow-acting, and expensive. Overall, instead of fixing the problem, we only control the symptoms. And that leads to poor compliance – so, in many cases, the disease wins in the end.
SEECASE, however, was very interesting; by restricting recruitment to those with highly symptomatic disease, the trial set the efficacy bar quite high. The broad and significant improvement observed in the SEECASE treatment group therefore is extremely encouraging: we saw reduced severity and frequency of burning, stinging and dryness; reduced awareness of symptoms; and decreased corneal fluorescein staining. I was particularly struck by the proportion of patients willing to purchase the treatment they were receiving after the clinical trial was finished – on this measure, NOV03 had double the satisfaction rates of other products I
have investigated. So my impression is that patients are extremely happy with NOV03.
In conclusion, clinical trials of DED products very rarely show simultaneous improvement in symptoms and signs – and it’s even more uncommon to see improvements of the magnitude found in SEECASE. With data like these, we should be optimistic regarding the likely impact of NOV03 in DED associated with MGD.

SEECASE Phase II – Aiming High
This randomized, double-masked, multicenter US trial (n=336) enrolled highly symptomatic patients:
• low TBUT (5 seconds or less)
• MGD score 3 or more
• normal Schirmer ~15 mm
• OSDI ~55
• mild to moderate corneal damage
For more information regarding NOV03, visit www.novaliq.com
References
- P Agarwal et al., “Preclinical studies evaluating the effect of semifluorinated alkanes on ocular surface and tear fluid dynamics”, Ocul Surf, pii: S1542-0124(18)30322-7. PMID: 30831252. S Kroesser et al, “Ocular and systemic distribution of 14C-perfluorhexyloctane following topical ocular administration to rabbits”, Poster board A0383, ARVO, May 2018. M Krafft and J Riess, “Chemistry, physical chemistry and uses of molecular fluorocarbonhydrocarbon diblocks, triblocks and related compounds – unique ‘apolar’ components for self-assembled collid and interface engineering”, Chem Rev, 109, 1714 -1792 (2009). PMID: 19296687. P Steven et al., “Semifluorinated alkane eye drops for treatment of dry eye disease due to meibomian gland disease”, Ocular Pharmacol Ther, 33, 678-675 (2017). PMID: 28922088. G Garhofer et al., “Influence of perfluorohexyloctane-containing eyedrops on tear film thickness in patients with mild to moderate dry eye disease”, Poster board B0119, ARVO, April 2018. Novaliq data on file: “NOV03: Better efficiency for better symptomatic relief in evaporative DED patients”, March 2019.
