Epithelium-off (epi-off) corneal collagen cross-linking (CXL) has been effective in stabilizing keratoconus, offering hope to many patients with this progressive condition (1). Epithelium-on (epi-on) CXL, in which the epithelium is minimally disturbed, has historically not been as successful, but clinicians and researchers continue to explore this option, believing it may reduce the risk of infection and corneal haze (2).
Although the risk of these complications in epi-off procedures is low (1, 3), striving to further improve safety is always a worthwhile goal. In addition, patients undergoing an epi-on procedure may experience less postoperative discomfort and faster visual recovery.
Various attempts have been made to modify established cross-linking protocols to make an epi-on version more effective. The complex biochemistry of CXL, in which riboflavin, ultraviolet (UV) light, and oxygen all play a role, forces us to confront several challenges. The intact epithelium blocks the transfer of riboflavin into the stroma and can absorb some of the UV light that must reach the stroma.
Furthermore, the epithelium blocks atmospheric oxygen from reaching the stroma and biomechanically reduces the amount of available oxygen. The chemical reactions in CXL procedures — both epi-on and epi-off — also deplete atmospheric oxygen instantly, reducing stromal oxygen to very low levels. Pulsing, or cycling the UV light on and off to allow oxygen to replenish during the “off” cycles, is helpful, but has not been sufficient to restore oxygen to the desired levels (4).
To perform epi-on CXL successfully, we ideally want to optimize all of the factors that we can control, including the riboflavin formulation (riboflavin, vehicle and other components), the intensity and delivery mode of the UV irradiation, and the delivery of supplemental oxygen.
Currently, several new riboflavin formulations designed to facilitate epi-on CXL are under investigation (5, 6, 7). Early work on sustained release riboflavin and nanotechnology formulations has also been reported (8). One promising new strategy is to increase the ambient oxygen concentration on the cornea by delivering supplemental oxygen at the ocular surface throughout the CXL procedure.
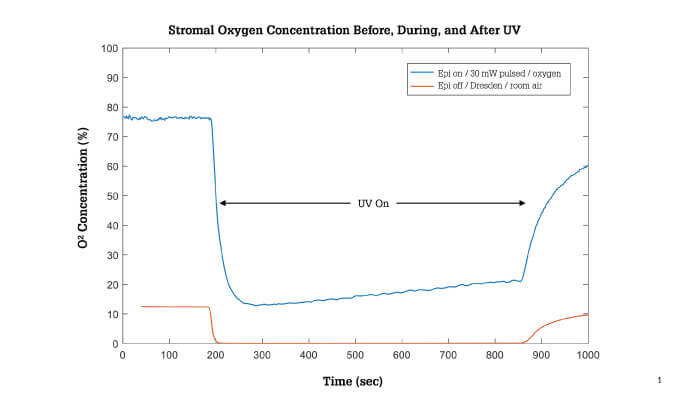
Recent laboratory research has demonstrated that this approach significantly increased the oxygen availability in the stroma of ex vivo porcine eyes treated with high-irradiance epi-on CXL compared to a pulsed protocol with no supplemental oxygen (see Figure 1) (9).
When researchers compared two epi-on protocols, again in an animal model, a greater degree of corneal collagen stiffening occurred in eyes receiving riboflavin with benzalkonium chloride (BAC), supplemental oxygen, and pulsed UV at 30 mW/cm2, with 1 sec:1 sec pulsing, and 10 J/cm2, compared with eyes that received riboflavin with sodium iodide, room air, and 4 mW/cm2, 15 sec:15 sec pulsing, and 4.1 J/cm2 UV (5). In my opinion, supplemental oxygen has the potential to improve both epi-on and epi-off CXL.
The scientific support for oxygen in cross-linking is currently being evaluated in Phase III clinical trials of epi-on CXL. I am a clinical investigator in this prospective, randomized, controlled study of a drug-device combination product from Avedro. In this study, several variables have been modified from traditional epi-off protocols.
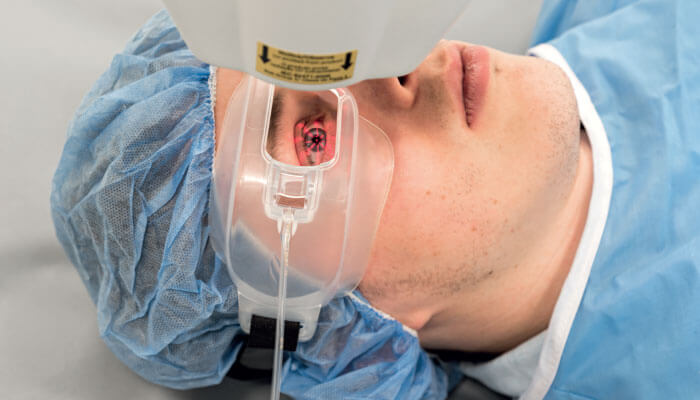
We are applying riboflavin that has been modified to enhance penetration through the epithelium into the stroma, delivering pulsed UV light at increased intensity, and providing supplemental oxygen using Boost goggles (see Figure 2). The goggles are worn throughout the UV light application, increasing the oxygen concentration on the corneal surface to >90 percent.
Enrollment in the study is now complete, with 275 patients with progressive keratoconus treated across 14 sites. Two-thirds of the patients receive the investigational epi-on CXL treatment, and the other one-third receive a sham treatment. When introducing this procedure to patients, I have explained that it is experimental. However, the risk for the patient is very low. After six months of follow-up, the sham control group is eligible to receive treatment; rescue treatment is also available during the initial 6 months, if needed.
Although some clinicians in the US already perform epi-on CXL procedures off-label or with unapproved devices, I believe it is important to wait for the clinical trial results. We know that the currently approved epi-off CXL treatment is effective and is considered the standard of care.
Before starting to offer epi-on to patients, we need to evaluate the safety and efficacy of new techniques in well-controlled clinical trials in humans. The results may help us determine which cases will benefit from epi-off or epi-on procedures and help us develop better treatment algorithms.
Cross-linking has been a wonderful advancement for our patients with keratoconus. There is still room for improvement, and I am enthusiastic about the potential for new protocols that modify more of the variables we can control to achieve the greatest efficacy possible with an intact epithelium. In addition to the oxygen innovations described here, I hope that future protocols may allow us to customize the application of UV light.
Until now, in the US we have delivered a broad beam uniformly across the cornea. Based on studies performed throughout the world, it seems likely that we will achieve more effective treatments by treating different parts of the cornea with different levels of irradiance. Perhaps we will even be able to improve vision in patients with keratoconus, not just halt its progression.
References
- PS Hersh et al., “United States Multicenter Clinical Trial of Corneal Collagen Crosslinking for Keratoconus Treatment”, Ophthalmology, 124, 1259-70 (2017). PMID:
- N Soeters et al., “Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: a randomized controlled trial”, Am J Ophthalmol, 159, 821-8 (2015). PMID:
- D O’Brart, “Corneal collagen crosslinking for corneal ectasias: A review”, Eur J Ophthalmol, 27, 253-69 (2017). PMID:
- L Sun et al., “Transepithelial accelerated corneal collagen cross-linking with higher oxygen availability for keratoconus: 1-year results”, Int Ophthalmol, 38, 2509-17 (2018). PMID:
- J Hill et al., “Biomechanical impact of drug formulation, supplemental oxygen, and UV delivery on epi-on CXL”. Presentation at ARVO, April 27-May 2, 2019, Vancouver, Canada.
- C Ostacolo et al., “Enhancement of corneal permeation of riboflavin-5’-phosphate through vitamin E TPGS: A promising approach in corneal trans-epithelial cross linking treatment”, Int J Pharm, 440, 148-53 (2013). PMID:
- R Stulting et al., “Corneal crosslinking without epithelial removal”, J Cataract Refract Surg, 44, 1363-70 (2018). PMID:
- R Rubinfeld et al., “Corneal cross-linking: The science beyond the myths and misconceptions”, Cornea, 38, 780-90 (2019). PMID:
- J Hill et al., “Stromal oxygen dynamics during high-irradiance epi-on corneal crosslinking”. Presentation at ARVO, April 27-May 2, 2019, Vancouver, Canada.
