Blindness induced by corneal disease continues to be a huge public health burden, with estimates that it affects around 1.5 million people worldwide (1). Corneal transplantation is an effective treatment option for many patients and has a high success rate in restoring sight, but there’s a catch: the severe shortage of donor corneas means that demand far outstrips supply. And even when donor corneas are available, contamination poses a real risk (2), further reducing the amount of viable tissue available.
Much research has focused on improved methods for early corneal disease diagnosis, as well as on advanced surgical methods that optimize our use of the tissue that we have available to us. With that in mind, one such surgical method, pre-Descemet’s endothelial keratoplasty (PDEK), is effective regardless of donor age (3, 4, 5).
Simply put, the PDEK technique transplants the pre-descemet’s layer (PDL) along with descemet’s membrane (DM) endothelium, which are separated from the residual donor stroma through the formation of a type-1 big bubble (BB). Here we describe the technique, which not only minimizes the use of donor tissue, but it can also be applied using adult and infant donor tissue, too, making it an important one for corneal surgeons to master.
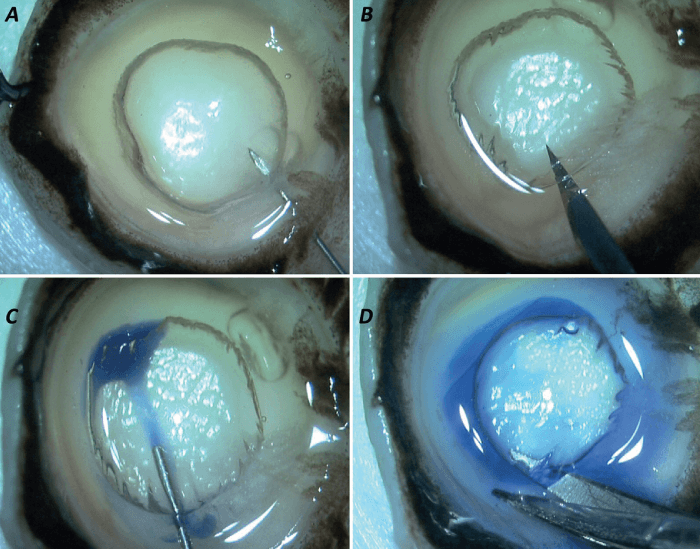
The graft can be obtained from a dissected corneoscleral button; the donor corneoscleral rim is placed on the eye mount with the endothelial side facing up.
The creation of a type-1 BB is essential to this procedure. To do so, a 30-gauge needle is used, which is attached to an air-filled, 5 ml syringe (Figure 1a). The needle is introduced with a bevel up position from the periphery up to the mid-peripheral area at a considerable depth from the DM so as to create a plane of separation between the PDL and the residual stroma.
Worthy of note is that the air dissection is carried out manually by the surgeon, so it has the advantage of being cost-effective. It is typically dome-shaped and spreads from center to periphery, with its diameter usually varying from 7.5-8.5 mm. Importantly, this type of BB never extends to the extreme periphery to avoid any adhesions between the PDL and the residual stroma. Injection of air causes the PDL-DM-endothelium complex to separate from the residual stromal bed (see Figure 1b).
For improved visualization, the graft is stained with Trypan blue, which is injected using a side port blade at the extreme periphery of the BB (Figure 1c). The BB is then cut across the periphery using corneoscleral scissors and is placed in the storage media (see Figure 1d).
The PDEK procedure is performed under local anesthesia. In cases of bullous keratopathy, the initial step comprises scraping and debridement of epithelium to enhance the intraoperative view. An anterior chamber maintainer (ACM) or a Trocar ACM is introduced into the eye to help maintain adequate anterior chamber (AC) depth at all times, while also ensuring a smooth transition between air and fluid infusion, as and when required.
A 2.8 mm corneal tunnel is made and two side port incisions are framed. With the AC completely inflated with air, the DM is scored and stripped using a reverse Sinskey hook. Inferior iridectomy is then performed, using a vitrectomy probe introduced from the corneal incision, which prevents pupillary block at a later stage.
The graft is held gently with non-toothed forceps and is placed into the cartridge of a foldable IOL filled with balanced salt solution. Air infusion is paused and the graft is gently injected into the AC through a clear corneal incision, taking extra care to avoid wound-assisted implantation. The orientation of the graft is verified before being gently unfolded using air and fluidics. Corneal indentation and massaging are performed to facilitate the graft unrolling.
Once the graft has partly unrolled, a small air BB is injected beneath the graft, which helps it to adhere to the corneal surface. The peripheral edges of the graft can then be unrolled by gently manipulating it with a reverse Sinskey hook; once it has fully unrolled, air infusion is initiated to facilitate complete adherence of the graft to the recipient bed. A well-formed AC is then achieved using corneal sutures and a complete closure of all wounds.
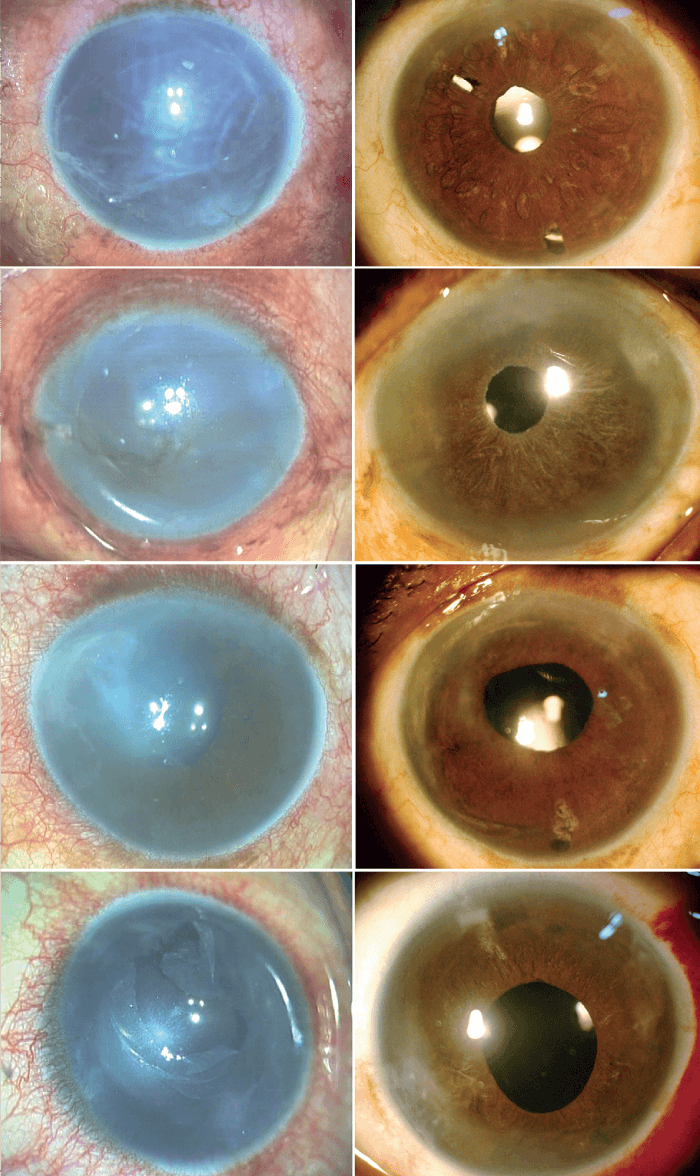
Given our experience to date with this procedure, we predict that PDEK will have widespread acceptance. It is associated with rapid visual recovery, predictable wound strength, and good optical predictability, much like DMEK (see Figure 2). However, PDEK has some important added advantages: i) better graft maneuverability, resulting from the extra strength and splinting effect of the PDL to the DM-endothelium complex, ii) reduced risk of donor tissue loss. Furthermore, PDEK does not require greater financial investment.
Other differences between the two procedures include the thickness of the donor graft; PDEK involves the addition of the PDL, increasing the thickness of the graft to approximately 25-30 µm, which is comparatively thicker than that of the DMEK that is 10-15 microns, but is thinner when compared with DSEK/DSAEK ( 100-200 microns) or an ultra-thin DSAEK (UT-DSAEK) – approximately 90-100 microns thick.
Visual rehabilitation after PDEK is faster than DSEK and its variants. In our experience as the graft is thinner than in DSEK, it takes comparatively less time to establish endothelial functionality, as less stroma is involved in the graft. Finally, the fact that it can be feasibly performed using both infant and young donor grafts is, of course, hugely advantageous. With the current supply of donor tissue worldwide ever-decreasing, expanding the pool of appropriate cornea donor tissue will become an increasingly important benefit.
References
- D Pascolini and SP Mariotti, “Global estimates of visual impairment: 2010”, Br J Ophthalmol, 96, 614 (2012). PMID: 22133988.
- R Vignola et al., “Monitoring the microbial contamination of donor cornea during all preservation phases: A prospective study in the Eye Bank of Rome”, Transpl Infect Dis, 21, e13041 (2019). PMID: PMID: 30582780.
- A Agarwal et al., “Pre-Descemet’s endothelial keratoplasty (PDEK)”, Br J Ophthalmol, 98, 1181 (2014). PMID: 24659352.
- HS Dua et al., “Human corneal anatomy redefined: A novel pre-Descemet’s layer (Dua’s layer)”, Ophthalmology, 120, 1778 (2013). PMID: 23714320.
- A Agarwal et al., “Pre-descemet endothelial keratoplasty with infant donor corneas: a prospective analysis”, Cornea, 34, 859 (2015). PMID: 26057329.
