- The CEDARS algorithm was created as a response to great numbers of patients being treated for ocular surface disease and dry eye
- Testing is used to get a diagnosis and develop a treatment plan, based on the diagnosis rather than severity
- Cryopreserved amniotic membrane tissue can be used to treat common corneal pathologies associated with OSD.
Years before the now ubiquitous “dry eye center of excellence” emerged as a pervasive practice model, I was treating large numbers of ocular surface disease (OSD) and dry eye disease (DED) patients and, like so many, recognized how complex this issue is. Treating these patients inspired the creation of the Cornea, External Disease, and Refractive Society (CEDARS) Dysfunctional Tear Syndrome Algorithm, which I developed along with my colleagues Kenneth Beckman and Jodi Luchs on behalf of the CEDARS Dysfunctional Tear Syndrome Panel.
The foundation of the CEDARS algorithm is that we base treatment on diagnosis rather than severity. The CEDARS algorithm separates dysfunctional tear syndrome into diagnostic categories (aqueous deficiency, blepharitis, and so), which then guide treatment. We use testing to arrive at a diagnosis and the diagnosis to guide our treatment plan, keeping in mind that the patient may have more than one diagnosis. Finally, we treat the coexisting pathologies with a logical, step-wise approach (1). The formula goes hand-and-hand with what I have learned from these challenging patients for more than two decades: i) OSD is a multifactorial disease that often requires multiple forms of treatment; and ii) patients typically get some relief with a first-line treatment, but it’s usually the second or even third intervention that achieves our treatment goals.
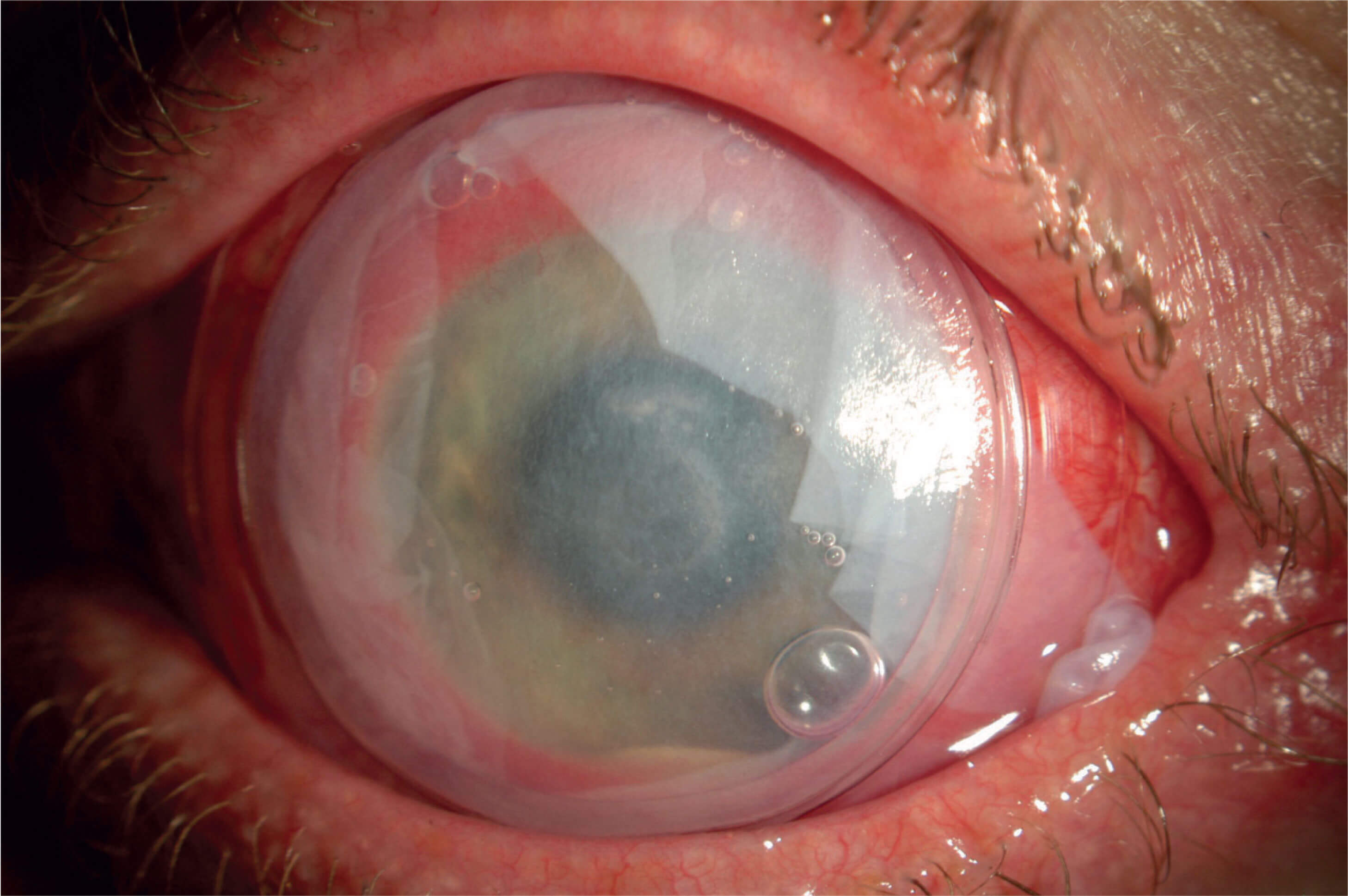
My success with cryopreserved amniotic membrane (CAM) tissue has led to its inclusion in the CEDARS algorithm. Throughout the years, I have seen CAM tissue evolve from a last-ditch initiative reserved for only the most recalcitrant OSD and DED patients – in other words, a treatment based solely on severity – to a quick and simple in-office procedure that is frequently relied upon to augment other therapies for patients at various stages of disease severity. CAM tissue is a staple in my practice; I use it to treat common corneal pathologies associated with OSD, such as superficial punctate keratitis, filamentary keratitis, recurrent corneal erosion, corneal ulcers, neurotrophic keratitis, neurotrophic ulcers (Figure 1), exposure keratitis, and Sjogren’s syndrome. In my hands, CAM provides these patients with relief for many months.
Frozen or dehydrated?
Not all amniotic membrane tissue is the same. The key distinction lies in how CAM tissue and dehydrated extracellular membrane derived from human amniotic tissue are processed. Cryopreserved AM (PROKERA, Bio-Tissue) is kept frozen and brought to room temperature just before placement. Dehydrated AM (AmbioDisk, IOP Ophthalmics/Katena, BioDOptix, DermaSciences, Aril, BlytheMedical) is stored at room temperature and must be rehydrated for clinical use. Cryopreservation allows the tissue to maintain the structural and biological integrity of the membrane by retaining the extracellular matrix components: heavy-chain hyaluronic acid (HC-HA)/pentraxin 3 (PTX3), which is associated with anti-inflammatory, anti-scarring, and regenerative properties (2). In contrast, dehydration breaks down HC-HA/PTX3 to pro-inflammatory low molecular weight hyaluronic acid, and the structural integrity is lost (3). I think of HC-HA/PTX3 as the “secret sauce” that adds exactly what is needed by complex OSD patients, who comprise a hefty portion of my patient base.
CAM has multiple benefits. First, it retains all the hard-working anti-inflammatory mediators that initiate regenerative healing. Second, it is “self-retained,” which means that it maintains placement on the cornea via a polycarbonate ring secured to the membrane. Dehydrated AM, on the other hand, needs a bandage contact lens (CL) to keep it in place.
In my experience, dehydrated tissue tends to get lost very quickly; sometimes it slips out from under the CL, and other times it crinkles up in the corner of the eye. The CL introduces other issues, such as increased risk of infection or hypoxia. In addition, there are certain OSDs that actually benefit from the polycarbonate ring of CAM; for example, with Stevens-Johnson syndrome or chemical injuries, the ring acts as a symblepharon ring to prevent scarring, which you do not get with the combination of dehydrated AM and a CL.
Reversing the Cycle
DED involves a defect in the neuronal feedback loop. DED patients have decreased corneal sensation, which initiates the process of shutting down the lacrimal gland. The lacrimal gland becomes inflamed and produces T-cells and cytokines; these inflammatory mediators are then secreted in the tears and end up damaging the ocular surface, which causes more of a neurotrophic effect and a decrease in nerve impulses. A message is sent to the brain that a problem exists, which shuts down the lacrimal gland and perpetuates the cycle.
By regenerating nerves, we can potentially reverse the cycle. Research suggests that in addition to stimulating active healing, CAM initiates corneal nerve regeneration (4, 5). In a prospective, controlled study comparing PROKERA Slim with conventional treatment in patients with moderate-to-severe DED, PROKERA Slim promoted a lasting effect by increasing corneal nerve density. The same study also showed rapid reduction of symptoms, pain, and corneal staining in patients treated with PROKERA Slim.
PROKERA’s lasting effect was reproduced in a larger retrospective study of 100 moderate-to-severe DED patients who were not responding to maximum conventional therapies; 88 percent demonstrated improved corneal staining scores along with a notable reduction in the severity of their DED symptoms (6).
More recently, in a small retrospective case series of patients who received PROKERA Slim or PROKERA Clear for acute treatment of neuropathic corneal pain, sustained pain control was demonstrated in 80 percent of treated eyes for more than 9 months after a single placement (7). And even when left in for only 6 or 4 days, there was a 72.5 or 63.1 percent reduction in pain, respectively. The fact that the researchers were still seeing improvement in nerve sensation and nerve regeneration at 9 months is impressive and coincides with what I have seen in my practice.
With studies touting results that match my own clinical experience, I am convinced that CAM is a game-changer when it comes to managing patients with OSD and DED.
Disclosures: Speaker consultant for Allergan, Bausch & Lomb, Shire, TearScience, Sun, Ocular Science, Bio-Tissue, Avedro, Omeros, Valeant, and EyeVance. Research performed for Kala, EyeGate, Biotherapeutics, Aldeyra, and Icare and has ownership interest in RPS, EyeVance, and Percept Corp.

References
- MS Beckman et al., “Dysfunctional tear syndrome: Dry eye disease and associated tear film disorders - new strategies for diagnosis and treatment”, Curr Opin Ophthalmol, 27 Suppl 1, 3-47 (2017). PMID: 28099212. SCG Tseng, “HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: insight into relationship between inflammation and regeneration”, Invest Ophthalmol Vis Sci, 57, 1-8 (2016). PMID: 27116665. M Cooke et al., “Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue”, J Wound Care, 23, 465-476 (2014). PMID: 25296347. AM Cheng et al., “Accelerated restoration of ocular surface health in dry eye disease by self-retained cryopreserved amniotic membrane”, Ocul Surf, 14, 56-63 (2016). PMID: 26387870. T John et al., “Corneal nerve regeneration after self-retained cryopreserved amniotic membrane in dry eye disease”, J Ophthalmol., 6404918 (2017). PMID: 28894606. MB McDonald et al., “Treatment outcomes in thy Eye Amniotic Membrane (DREAM) study”, Clin Ophthalmol., 12, 677-681 (2018). PMID: 29670328. M Morkin et al., “Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain”, 16, 132-138 (2018). PMID: 29032001.
