Ocular surface disease (OSD) is more widespread than many clinicians once thought. Recent studies have shown that the majority of patients presenting for cataract surgery have ocular signs of dry eye or Meibomian gland dysfunction (MGD), regardless of whether they report symptoms (1, 2). My colleagues and I have demonstrated that signs of MGD, once thought to be a condition limited to postmenopausal women, are common even in children (3).
Although we are fortunate to have a wide array of treatments for OSD, including artificial lubricants, topical anti-inflammatory drops, thermal pulsation, and punctal occlusion, many patients get insufficient relief from these approaches. There are a number of new and pipeline treatments designed to address the gaps in treatment.
In recent years, we have seen the development of several new higher-concentration formulations of cyclosporine. The only FDA-approved therapy so far is Cequa (Sun Pharma), which has nearly double the concentration of cyclosporine (0.09%) compared with the established drug, Restasis (cyclosporine 0.05% Allergan).
To me, what is even more important than the concentration is Cequa’s novel nanomicellar formulation, which likely aids absorption and tissue penetration by improving the drug’s bioavailability. As a result, improvements in Schirmer scores and corneal staining were seen as early as one to three months in Phase III clinical trials (4), which is more rapid than we have come to expect from topical cyclosporine.
We can also expect to see a new topical loteprednol formulation on the market soon. Positive results were reported from the Phase III STRIDE-3 trial of KPI-121 (loteprednol 0.25%, Eysuvis, Kala Pharmaceuticals) and the company has just submitted a new drug application for this drug with the FDA. Patients treated with the low-dose steroid had improvements in ocular discomfort severity and conjunctival hyperemia.
Here again, the formulation – a mucous-penetrating particle drug delivery technology meant to enhance delivery – may play an important role. I would expect to use this new drug to treat patients with episodic flares of dry eye, for whom we don’t currently have good options. I’m reluctant to give these patients a 15-ml bottle of prednisolone due to the potential for misuse, whereas loteprednol has an excellent safety profile.
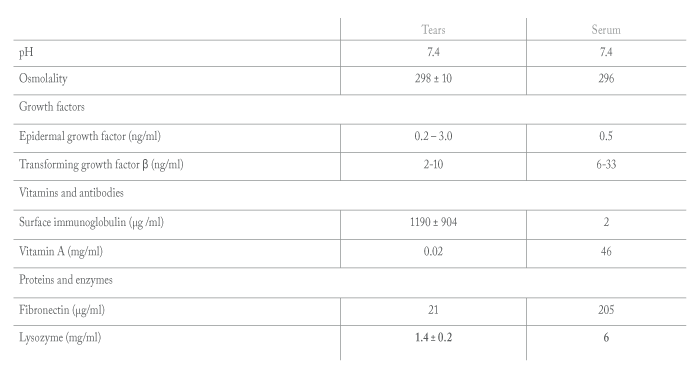
Cornea specialists have long relied on autologous serum eye drops (ASEDs) for highly symptomatic dry eye patients and those with significant staining or poor epithelial healing. Like natural tears, serum contains many replenishing growth factors that are not available in any other dry eye treatment modality (See Table 1) (5).
However, the rate-limiting factor preventing wider adoption of ASEDs has been the difficulty of obtaining them, especially for anyone outside of a major city or research university. Patients had to get their blood drawn, then work with the physician to deliver the blood to a compounding pharmacy able to produce the serum.
In recent years, a company called Vital Tears has greatly simplified the process for both doctors and patients, with faster turnaround and lower cost. Vital Tears collaborates with a large network of labs across the country and centralizes preparation and shipping of the drops. Patients can now get their blood drawn in their home town (or sometimes even in their own home) and then Vital Tears takes care of the rest.
This makes it much easier to prescribe ASEDs for our patients with moderate to severe dry eye. I’ve also found them to be invaluable for patients with multiple sensitivities and allergies who can’t tolerate preservatives or other components in commercial drops.
Stimulation of the trigeminal nerve through the nose is a novel treatment pathway that was first introduced by Allergan, with its TrueTear device. The device is placed in the intranasal cavity to deliver a mild electric current that stimulates the trigeminal nerve (CN V), which is responsible for innervation of the lacrimal functional unit. In addition to triggering lacrimation through the efferent pathway, it also stimulates the Meibomian glands and goblet cells to produce complete, physiological tears (6, 7). However, accurate insertion and positioning can be challenging, limiting effective use of the device.
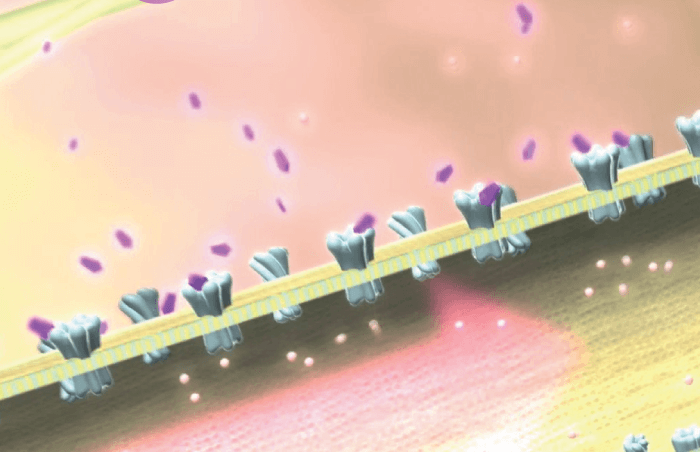
That barrier may be overcome by a nasal spray technology currently in the pipeline (OC-01, Oyster Point). This preservative-free spray contains a nicotinic acetylcholine receptor (nAChR) agonist to stimulate the trigeminal nerve parasympathetic pathway (Figure 1). It was just announced that primary and secondary endpoints were successfully met in the drug’s Phase III clinical trial, ONSET 2, so we can expect to see a new drug application submitted later this year. The novel mechanism of action and rapid onset could make OC-01 a nice complement to existing drug options.
A common cause of blepharitis is the Demodex folliculorum mite, which has proved challenging to treat. Tarsus Pharmaceutical is developing a preserved, multi-dose topical drop with a new molecule (TP-03) that – when used twice daily for six weeks – results in the paralysis and death of the Demodex mites. A Phase II study revealed positive results in decreasing both the mite load and the collarettes or cylindrical dandruff that are pathognomic for Demodex blepharitis. A Phase III trial is launching soon and Phase II trials for MGD and rosacea are underway.
Further out in the pipeline, but certainly on my wish list, are treatments for corneal pain. There are a number of new molecules under investigation for pain caused by severe dry eye or neuropathic conditions masquerading as dry eye. For example, Neuroptika, a company spun off by Senju Pharmacuetical, has a new small-molecule topical drug, NRO-1.
It releases glial cell-derived neurotrophic factor (GDNF) to protect and regenerate corneal nerves. Researchers hope it will improve corneal sensitivity, lacrimation, and corneal epithelial health in patients with dry eye and other conditions. It has shown some promising results in animal studies and is currently in Phase II human clinical trials. At the University of Illinois at Chicago, researchers are studying an enzyme-based treatment for severe dry eye. In this approach, drops containing recombinant deoxyribonuclease (DNase) are used to break up pro-inflammatory DNA material on the ocular surface. A Phase I/II study showed the treatment to be effective in improving corneal damage and reducing corneal pain in patients with Sjögren’s syndrome or graft-vs-host disease (8).
It’s exciting to see so many new avenues opening up in dry eye treatment, from unique formulations of older drugs to innovations in drug delivery and logistics – even new mechanisms of action altogether.
References
- WB Trattler et al., “Cataract and dry eye: Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO)”, Clin Ophthalmol, 11, 1423 (2017). PMID: 28848324.
- B Cochener et al., “Prevalence of Meibomian gland dysfunction at the time of cataract surgery”, J Cataract Refract Surg, 44, 144 (2018). PMID: 29587971.
- PK Gupta et al., “Prevalence of Meibomian gland atrophy in a pediatric population”, Cornea, 37, 426 (2018). PMID: 29286952.
- DF Goldberg et al., “A phase 3, randomized, double-masked study of OTX-101 ophthalmic solution 0.09% in the treatment of dry eye disease”, Ophthalmology, 126, 1230 (2019). PMID: 30965064.
- G Geerling et al., “Autologous serum eye drops for ocular surface disorders”, Br J Ophthalmol, 88, 1467 (2004). PMID: 15489495.
- G Dieckmann et al., “In vivo confocal microscopy demonstrates intranasal neurostimulation-induced goblet cell alterations in patients with dry eye disease”, Poster, ARVO, May 7-11, Baltimore, USA (2017).
- M Watson et al., “Effect of the intranasal tear neurostimulator on Meibomian glands”, ARVO, May 7-11, Baltimore, USA (2017).
- C Mun et al., “A phase I/II placebo-controlled randomized pilot clinical trial of recombinant deoxyribonuclease (DNase) eye drops use in patients with dry eye disease”, Transl Vis Sci Technol, 8, 10 (2020). PMID: 31110911.
