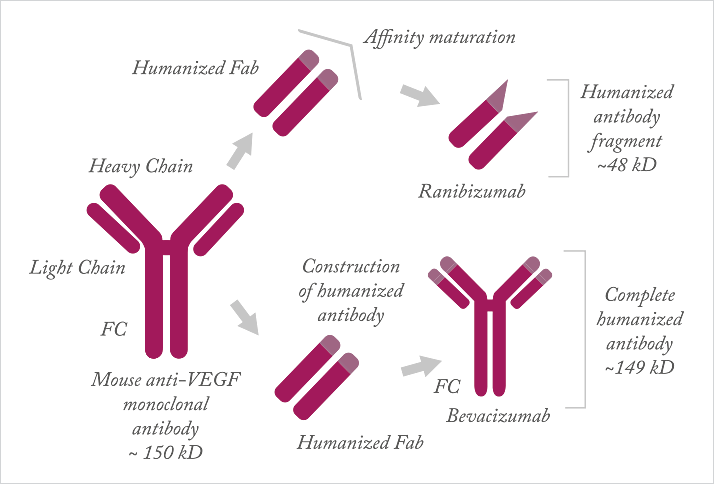
There’s a fair amount of evidence out there that supports the use of Roche’s VEGF inhibitor, bevacizumab for the treatment of wet AMD (AMD). Around 60 percent of patients with wet AMD in the US receive it, but for most of the world, such use is off-label.
In the UK’s National Health Service (NHS), one dose of ranibizumab costs £740, whereas a single dose of bevacizumab comes in at least a tenth of the cost. In 2009, against that background, the NHS was planning to perform its own clinical trials, in order to get evidence regarding the relative safety and efficacy of ranibizumab and bevacizumab in wet AMD: the IVAN (head-to-head comparison [2]) and TANDEM (dose-finding [3]) trials. It’s at this point where an investigation by the BMJ (4) alleges that Novartis tried to interfere with the operation of these trials. The key allegations are:
- Novartis tried to prevent UK ophthalmologists joining the IVAN trial, “with their sales representatives lobbying potential principal investigators against the trial and telling them that the IVAN protocol was seriously flawed”
- Novartis urged some primary care trusts to pull out of the trial, hinting that industry funding would be lost for other trials
- A Novartis sales representative warned Alex Foss, TANDEM’s principal investigator, that “Novartis would do everything it could to stop the TANDEM trial and would particularly challenge its ethics,” and that “that the challenge would not come from Novartis itself but from the RNIB [Royal National Institute of Blind People]”
- The RNIB did indeed express concerns about the design of TANDEM one year later, in 2010 – and the person that raised those concerns now works for Novartis Oncology.
Novartis refute these allegations, with a spokesperson telling the BBC that they “take any allegations seriously and are closely reviewing the content of the article,” adding that “Novartis is committed to improving health outcomes for patients with serious eye disease as demonstrated by our substantial engagement in ophthalmology, including significant research and development efforts in the UK” (top.txp.to/0415/bbc-health). The spokesperson made the point that “Novartis continuously conducts clinical trials in the UK and other countries. Discussions with UK study sites and healthcare professionals occur during all stages of these clinical trials,” explaining that, “During the feasibility stage these discussions often focus on the capacity and capability to conduct studies in accordance with the highest clinical and ethical standards.” The RNIB also robustly challenged the BMJ’s assertions (top.txp.to/0415/rnib-BMJ), stating that “The reason we raised an ethical challenge to the TANDEM trial is because we believe it takes unnecessary risks with patients’ eyesight, which could ultimately lead to them going blind. Wet AMD can develop extremely quickly, sometimes leading to complete sight loss within three months. The design of the TANDEM trial means that some patients might receive dosages which are so low they won’t be effective, by this point it could be too late to save their sight. We feel strongly that this is unacceptable.”
References
- N Dickinson, “Response to BMJ article about unlicensed prescribing of Avastin for AMD”, bit.ly/BMJGMC. Accessed April 4, 2015. U. Chakravarthy et al., “Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial”, Ophthalmology, 119, 1399–411 (2012). PMID: 22578446. A Foss et al., “Comparing different dosing regimens of bevacizumab in the treatment of neovascular macular degeneration: study protocol for a randomised controlled trial”, Trials, 16:85 (2015). D Cohen, “Why have UK doctors been deterred from prescribing Avastin?”, BMJ, 350, h1654. (2015). PMID: 25834024.
