- Approaches for diagnosing and treating pediatric and adult intraocular and ocular surface tumors differ, but advanced techniques are increasingly being used for both patient groups
- Treatment approaches include brachytherapy, proton beam radiotherapy, photodynamic therapy (PDT), and chemotherapy depending on the type, size and location of the tumor
- For pediatric retinoblastoma, local cryotherapy or laser treatment and brachytherapy plaques can be used for smaller tumors; chemotherapy approaches are used for more advanced cases, given systemically, into the ophthalmic artery or vitreous cavity.
- Application of virtual clinics and artificial intelligence has the potential to pick up suspicious lesions at an earlier stage – and to make the referral process much more streamlined.
The eye can develop a range of primary or secondary tumors: some are more common in adults, while others are more typical of children. We therefore adopt somewhat different investigational approaches for the pediatric and adult populations; in both groups of patients, taking a detailed medical and ophthalmic history, performing a thorough examination and using imaging are key to correct diagnosis and management. These patients are best managed as part of a multi-disciplinary team of professionals.
Grown-up work-up
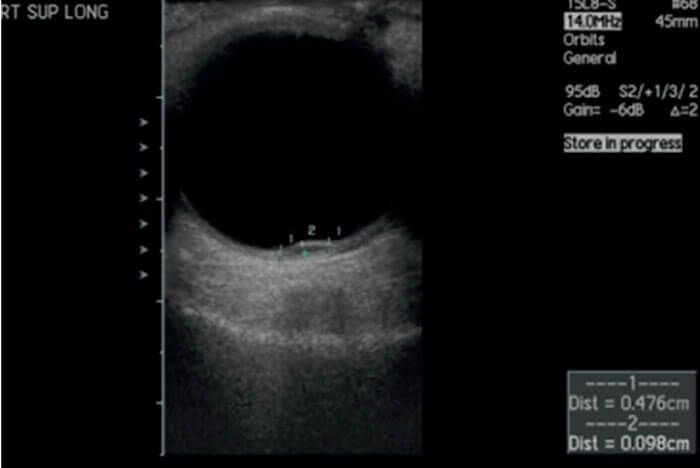
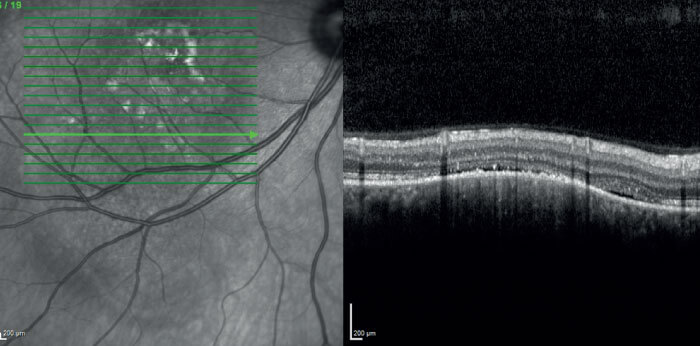
In adults, we deal with diverse tumor types, from benign choroidal nevi to malignant melanoma; vascular tumors in the fundus that can be part of a syndrome; lymphoma; and secondary deposits in the eye. We also see conjunctival tumours such as melanoma and its precursor, primary acquired melanosis. When investigating adult melanoma suspects, a large part of our diagnostic work-up comprises procedures aimed at distinguishing benign from malignant or pre-malignant tumors. The need for this differentiation is a consequence of the high incidence of benign lesions – up to 10 percent of the Caucasian population has a choroidal nevus. Furthermore, as imaging procedures become more sophisticated, increasing numbers of nevi are detected by optometrists and referred to ophthalmologists. How should we manage this burgeoning workload? At Moorfields, we think the answer includes the application of virtual clinics and artificial intelligence; our experience is that these tools help to efficiently differentiate benign nevi from melanoma in a large proportion of cases. The virtual approach makes assessment far less labor-intensive, and as it is still based on the Wills Eye Hospital clinical factors scheme (see sidebar “Know Your Nevi”), it should be no less rigorous as a screening method than the standard approach. Indeed, the prospective studies we have so far completed indicate that remote assessment of patients’ images – ultrasound scan, OCT scan and photograph – at a reading center gives results highly concordant with the gold standard (assessment by an ophthalmologist). These results are sufficiently encouraging for us to actively contemplate pushing this system back into the community. For example, by giving optometrists a scoring system so they have a better idea of what they should refer on to us, we could release specialists from the burden of assessing large numbers of nevi, which in the majority are harmless; remember, the risk of a nevus developing into malignant melanoma is only ~1 in 8,000.
Know Your Nevi – updating the features to incorporate imaging
Researchers at the Wills Eye Hospital have a long history in the refinement of systems for predicting the transformation risk of choroidal nevi. A 1995 study (2) identified five factors predictive of transformation into malignant melanoma:
- thickness greater than 2 mm on ultrasound scan
- fluid beneath the retina
- symptoms
- orange pigment
- margin of tumor.
These could be recalled with the assistance of the mnemonic “To Find Small Ocular Melanoma,” and the presence of three or more of these risk factors indicated a >50 percent chance of transformation.
The TFSOM algorithm has been of great practical assistance to clinicians, and has been updated at various points over the years, culminating most recently in the incorporation of risk factors as visualized with multimodal imaging (3). This new scheme can be remembered with the mnemonic “To Find Small Ocular Melanoma Doing IMaging,” and comprises the following:
- thickness >2 mm by ultrasound
- fluid in subretinal space by OCT
- symptoms of vision loss
- orange pigment by autofluorescence
- melanoma hollowness by ultrasound
- DIaMeter >5 mm by photography.
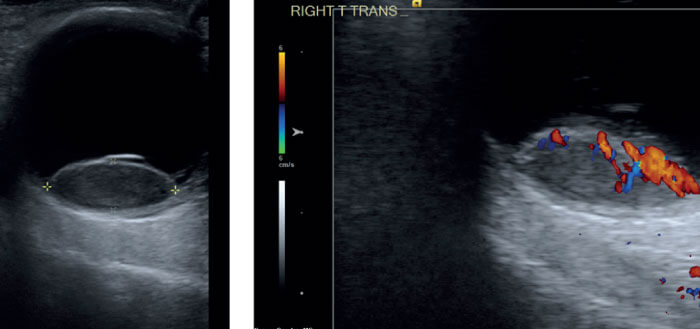
But for those lesions that do turn out to be malignant melanoma, what kind of management strategy should we apply? Again, clinical decisions rely heavily on imaging modalities because, unlike other sites in the body, we rarely take diagnostic biopsies from ocular tumors. Ultrasound technology is improving, but for key information we increasingly rely on OCT and auto-fluorescence. At Moorfields we also heavily employ ultrasound color flow mapping, which helps categorize the tumor; pattern recognition is a very important component of this technology.
Once we understand the tumor, the multidisciplinary team – comprising an oncologist, specialist nurses and several consultants – ratifies a management plan, with the patient at the center of the decision. Quite often, this includes a brachytherapy technique pioneered here at Moorfields and St Bartholomew’s Hospital in the 1920s and 30s: briefly, a small radioactive disk is sutured onto the eye so that the intraocular tumor is locally irradiated. Some tumors, however, are not suitable for brachytherapy – the tumor may awkwardly located or too big, for example. In those cases, we might administer proton beam radiotherapy. We’ve also been looking at the potential of photodynamic therapy (PDT) in the treatment of small melanomas – this approach may be able to eradicate the tumor with less vision loss than is associated with radiotherapy. I think we’ll see more of PDT type treatments in the future, as there are some interesting new drugs in development.
Some patients, however, have tumors that have progressed too far for radiotherapy to be of any use; in those cases, we must remove the eye. Fortunately, we have access to orbital implants, which can be attached to the orbital muscles, so that the false eye moves quite realistically. Even so, at Moorfields we continue to look for better ways of doing things, and currently we are investigating new technologies for rehabilitating the socket. Technology does not stand still!
In all cases, given the small risk of local (ocular) and larger risk of systemic relapse, melanoma patients need to be followed over the long term by an ocular oncologist and a medical oncologist. We know that certain melanoma genotypes are associated with a higher systemic relapse risk, so we offer appropriate genetic tests as necessary. In this context, we’re assessing next-generation sequencing; in particular, we are participating in a collaboration intended to sequence our ocular tumor tissue archives and identify correlations between genotype and patient survival.
Other adult tumors that we see include squamous cell tumors, lymphoid tumors, lymphoma, and benign reactive lymphoid hyperplasia. A new development in the management of these malignancies is the use of immunotherapy eye drops, such as interferon alpha, particularly in squamous cell tumors, such as ocular surface squamous neoplasia (OSSN). Equally, chemotherapy eye drops can be very useful; in fact, we recently carried out a study on the efficacy of 1 percent flurouracil eye-drops after surgical removal of OSSN. This work – which we carried out in Africa, where OSSN is relatively common – showed that a one-month course of these drops reduced OSSN recurrence threefold (1). It was particularly gratifying to be involved in this trial, because Africa has a higher prevalence of OSSN than the UK, but doesn’t have similar access to systems for reducing recurrence, such as devices to surgically freeze the edges of the resection margin or to give adjuvant radiotherapy. In resource-limited countries, a cheap chemotherapy eye drop can make a big difference to OSSN patients, so I like to think that Moorfields is making a global difference to eye cancer through this kind of work.
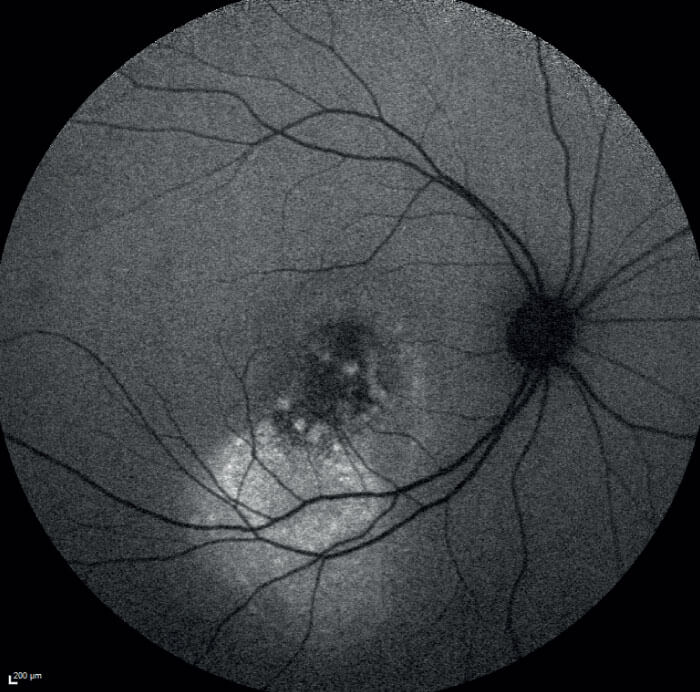
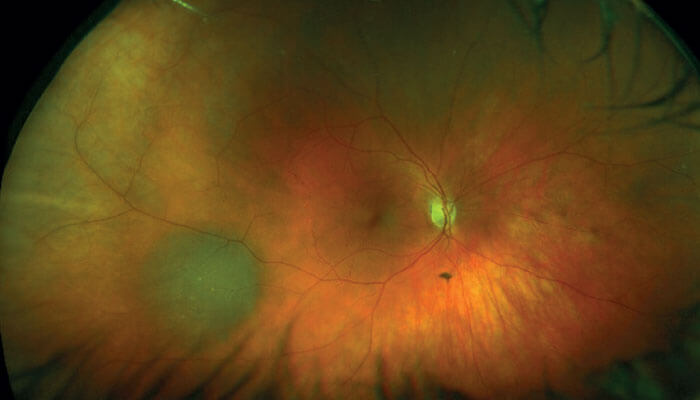
Kids’ stuff
Among pediatric patients, we see a number of retinoblastoma cases. This tumor is quite rare – only about 50 cases per year in the UK – and its identification requires a careful approach to differential diagnosis, which again relies heavily on imaging techniques (see sidebar “Reflect on the White Reflex”).
Where retinoblastoma is diagnosed, we can select from a range of treatments. For smaller tumors, we may opt for local cryotherapy or local laser treatment; brachytherapy discs also can be used for smaller retinoblastomas, but external beam radiotherapy, in which most or all of the eye is irradiated, is no longer used in retinoblastoma. For larger lesions, we may choose the systemic chemotherapy approaches developed in London about 30 years ago. However, a newer approach, developed in Japan and the USA, involves pulsing chemotherapy directly to the eye via a delivery catheter inserted at the groin and sent up through the neck to the ophthalmic artery. Many eyes that would otherwise be removed have been salvaged by this technique. A major source of failure in retinoblastoma treatment was vitreous seeding. Chemotherapy can also be applied locally in this situation, by intravitreal injection, but this approach was prohibited until recently due to concerns of seeding tumours outside the eye – a potentially life-threatening event. Recently, however, workers in Sweden and Switzerland developed a safety-enhanced technique for intravitreal chemotherapy, which avoids the release of tumor cells into the vitreous. The method involves reducing the IOP, administering the injection and freezing the needle track as the needle is withdrawn. Finally, for retinoblastomas that are too far advanced or that are resistant to treatment, enucleation may be the only choice. We are harnessing our archive of DNA from these patients to examine molecular signatures by next-generation sequencing, which may direct the choice of optimal treatment – hopefully entering the era of personalized medicine for eye tumours.
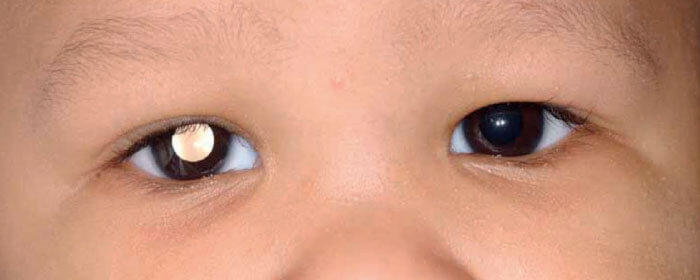
Reflect on the White Reflex
The white reflex – in which incident light causes a white reflection from the retina – is a classic signal of retinoblastoma. However, we should remember that the symptom does not correlate exactly with this condition; not all children with retinoblastoma have the white reflex, and not all children with a white reflex have retinoblastoma. Other conditions linked with this signal include the following:
- Normal finding – if the image captures the optic nerve
- Cataracts
- Congenital malformations, such as coloboma
- Retinal detachment
- Vascular diseases, such as retinopathy of prematurity, Coats’ disease or persistent fetal vasculature
- Non-retinoblastoma tumors, including medulloepithelioma or astrocytoma
- Inflammation; for example, as a consequence of ocular toxocariasis
- Vitreous hemorrhage following trauma
Observation of the white reflex in children therefore requires the ophthalmologist to rigorously apply differential diagnostics to distinguish between a range of serious conditions. That said, many of these conditions are very rare; one study suggests that among children with white reflex, the symptom is caused by cataracts in about 75 percent and retinoblastoma in about 20 percent of patients, while all other reported conditions occurred at frequencies <1 percent (4).
References
- S Gichuhi et al., “Topical fluorouracil after surgery for ocular surface squamous neoplasia in Kenya: a randomised, double-blind, placebo-controlled trial”, Lancet Global Health, 4, E378 (2016). PMID: 27198842.
- CL Shields et al., “Risk factors for growth and metastasis of small choroidal melanocytic lesions”, Ophthalmology, 102, 1351 (1995). PMID: 8719682.
- CL Shields et al., “Small choroidal melanoma: detection with multimodal imaging and management with plaque radiotherapy or AU-011 nanoparticle therapy”, Curr Opin Ophthalmol, 30, 206 (2019). PMID: 30844944.
- J Diagne et al., “Rare causes of childhood leucocoria”, J Fr Ophtalmol, 40, 676 (2017). PMID: 28893456.
