By Rubens Belfort, with Eduardo A. Novais, Andre Romano, Heloisa Nascimento and João Rafael Dias
For a retina specialist, accurately diagnosing and effectively managing conditions of the choroid is, in many ways, the “final frontier” of posterior segment diseases treatment. Understanding the nuances and impact of choroidal diseases is crucial to our understanding of broader intraocular health, however, until recently, our working knowledge of this pivotal area has been relatively limited by the imaging technologies available to us. Swept-source optical coherence tomography (SS-OCT) is a new and exciting technology that offers us the ability to better visualize the choroid, as well as other “deep” physiological structures in the posterior segment with remarkable clarity. SS-OCT is also advantageous when cataract, vitreous haze, hemorrhages, and other opaque media are present, as it can produce a high-fidelity image under challenging conditions. Commercially available SS-OCT technology uses a longer, ~1050 nm wavelength and has less variation in sensitivity with depth (sensitivity roll-off) compared with spectral-domain (SD) OCT. This improved immunity to ocular opacity and deeper penetration into the choroid (1, 2) allows improved visualization of the choroid, both on cross-sectional as well as en face OCTA imaging (3, 4).
In our practice, we currently use SS-OCT to image the choroidal area and differentiate etiologies in patients with uveitis, which is often difficult to accurately visualize when media opacity is present. Specifically, I have begun investigating the use of SS-OCT to image patients with suspected toxoplasmosis or viral retinitis; diseases that, given the additional information now available to us, I believe directly affect (or are affected by) the choroidal structure. Here, I will share some insights and interesting cases wherein SS-OCT offered me and my colleagues a detailed view of the posterior segment, and played a pivotal role in our increased understanding of disease manifestation. It is also worth noting that the use of SS-OCT has allowed us to explore different choroidal patterns in primary and secondary ocular infections, such as in patients with positive and negative IgM anti-toxoplasma circulating antibodies.
Differentiating between toxoplasmosis and viral retinitis
SS-OCT may be able to help clinicians quickly and confidently differentiate between toxoplasmosis and viral retinitis by analyzing the choroidal region in greater detail. As recently as 2017, research was done by Invernizzi and colleagues on the use of OCT technology to identify distinguishing characteristics of these diseases (5). However, intraretinal edema can often attenuate the OCT signal, masking choroidal features underneath this region. This issue is more common when using SD-OCT. With SS-OCT, we can acquire images with higher signal quality and make informed diagnostic decisions. SS-OCT has already shown us the significant role of the choroid in many so-called “classic” and “pure” retinitis cases.
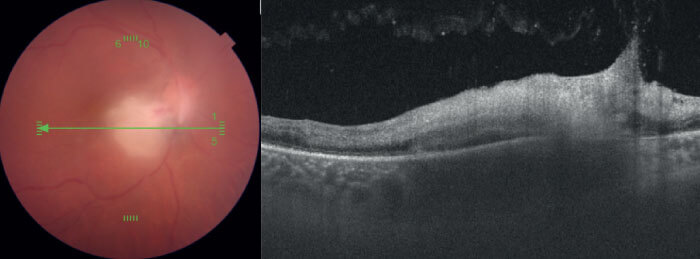
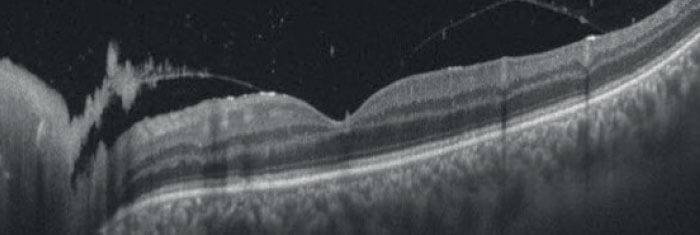
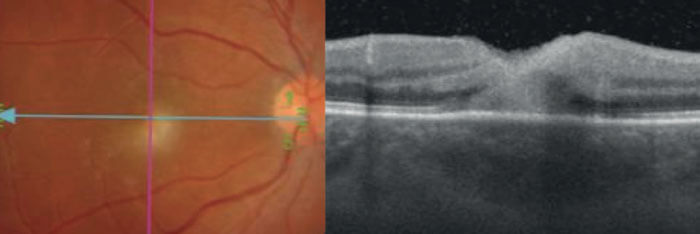
Specifically, two choroidal attributes, as identified by OCT scans, are associated with toxoplasmosis in the retinochoroidal region: hypo-reflectivity of the choroid beneath the necrotic focus, and the absence of hyperreflective septa within the choroid (see Figure 1). OCT scans from patients with viral retinitis, such as CMV and herpes, meanwhile, demonstrate that viral induced retinal necrosis does not affect underlying choroid (Figure 2). Although some degree of choriocapillaris alteration is present, the choroidal architecture is largely preserved. With this information in hand, it is much easier to make an informed diagnostic decision where the possibility of either disease is confirmed. Without a clear view of the choroid’s anatomy, it would be substantially more difficult to make this diagnosis with certainty, and manage the disease accordingly.
Identifying the role of the choroid in toxoplasmosis
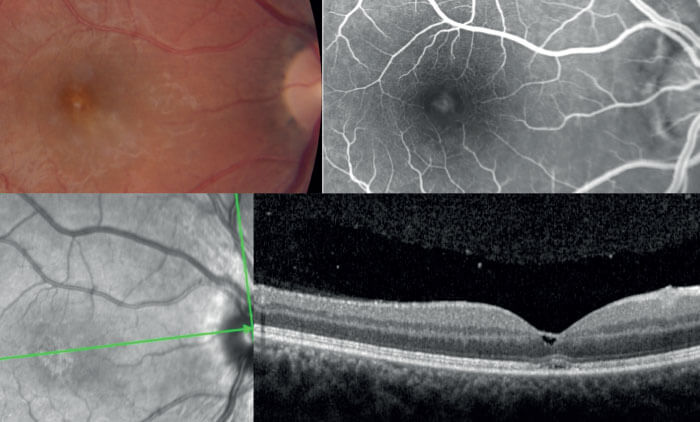
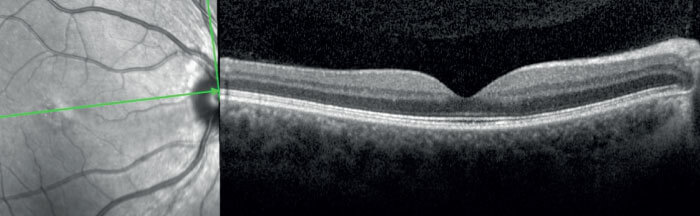
In addition to helping clinicians differentiate between disease states, the advanced imaging capabilities offered by new OCT technology can help us identify potential new associations and interactions during disease progression. For instance, a 29-year-old female was diagnosed with toxoplasmosis in April of 2018. She displayed a significant vitreous reaction, with a mild macular lesion on SD-OCT and a very small cyst in the fovea (see Figure 3). Her visual acuity was 20/40. The patient was treated and recovered, achieving 20/20 vision. Prophylactic course of anti-toxoplasmic medication to prevent recurrences was prescribed. At five months post-visit, the patient displayed no symptoms and scans appeared normal (see Figure 4). The patient decided to discontinue the treatment and, unfortunately, she presented a severe recurrence nine months later. She returned to our practice with 20/160 vision. Using SS-OCT (DRI OCT Triton from Topcon) to acquire multimodal imaging, we identified an abnormal thickness at the scleral-choroidal junction (see Figure 5). I now suspect that the choroid plays a larger role than previously thought in diseases that trigger cellular retinal infiltration. Fortunately, the deep tissue penetration made possible by swept-source technology creates new opportunities to understand the full range of physiological interactions present during posterior segment disease.
OCT-A application in toxoplasmosis
Unlike dye-based angiography, OCT-A is a non-invasive image modality that allows for the appreciation of depth-resolved spatial relationships of fundus vasculature. It also enables detailed en face visualization of the retinal and choroidal vessels separately, without the risk of adverse effects associated with the intravenous dye (6, 7). Choroidal neovascularization (CNV) is a potential complication that can cause severe visual loss in patients with posterior uveitis. Thus, the precise and early diagnosis of this sight-threatening disease is mandatory. The sensitivity of OCT-A to identify the neovascular complex may vary, as some clinical features such as hemorrhage, fluid, or fibrotic tissue may block OCT-A signal, preventing visualization of the CNV. However, as previously published by Novartis and colleagues, SS-OCT-A is better able to identify and demarcate the full extent of the abnormal vascular network (8). Thanks to this new technology we now have a powerful tool to better understand the natural history of CNV in posterior uveitis, avoiding the use of medications in many cases, as often symptoms disappear without interference.
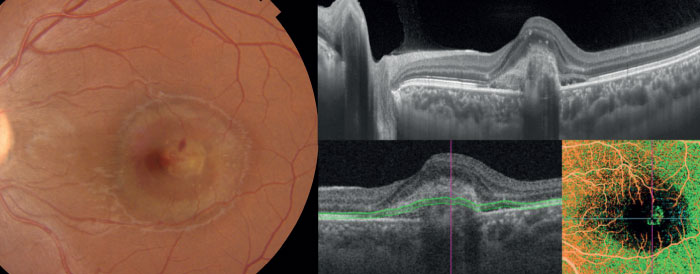
A specific case where SS-OCT angiography was valuable in this process involved an 18-year-old pregnant female patient who experienced vision loss of 20/200 in one of her eyes after recovering from toxoplasmosis, and was referred to us for the possibility of disease recurrence.
Using our SS-OCT device’s B-scan functionality, we discovered a subretinal hyperreflective lesion associated with subretinal fluid, and irregularity of the ellipsoid zone. A lack of vitreous cells and retinal edema, in conjunction with the presence of subretinal hyperreflective material, suggested the patient was suffering from a non-toxoplasmic infection. SS-OCT angiography clearly distinguished a type two choroidal neovascularization, and treatment with anti-VEGF was executed accordingly (see Figure 6).
Potential novel applications on the horizon
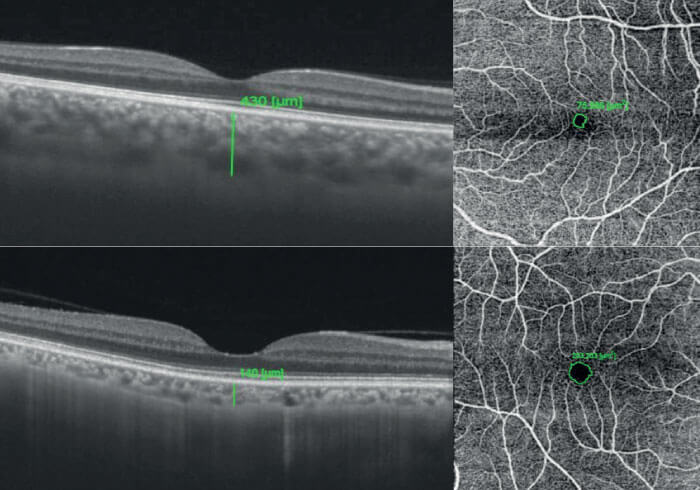
As SS-OCT and OCT-A are new technological developments, we are still in the pioneer stages of leveraging their capabilities to the fullest, and I expect that posterior segment researchers worldwide will continue to develop new and interesting ways to derive more clinical value from them. As an example, research presented at ARVO 2019 in Vancouver, Canada, demonstrated that patients with active inflammatory bowel diseases – such as Crohn’s disease and ulcerative colitis – frequently display a thicker choroid and smaller foveal-vascular zone (FAZ) during the active disease in comparison to the remission phase of disease (see Figure 7) using SS-OCT, which is able to render the choroid in great detail (9). In this study, many patients with active disease possessed 20/20 vision and showed no signs of inflammation. Without the image clarity offered by SS-OCT, researchers would have likely missed this key detail, along with the opportunity to identify this interesting association in autoimmune manifestation.
The arrival of new imaging technologies offers retinal specialists high-resolution images that are undoubtedly beautiful to behold. But it’s critical to note that while new imaging devices offer us beauty, they also offer us concrete clinical value – if we make the effort to use them in new and creative ways. Taking advantage of an unprecedented choroidal clarity, and the insights that come with it, is just one way to leverage the full potential of this new technology, and it is exciting to see how practitioners will continue to apply and derive value from SS-OCT.
References
- B Povazay et al., “Three-dimensional optical coherence tomography at 1050 nm versus 800 nm in retinal pathologies: enhanced performance and choroidal penetration in cataract patients”, J Biomed Opt, 12, 041211 (2007). PMID: 17867800.
- A Unterhuber et al., “In vivo retinal optical coherence tomography at 1040 nm - enhanced penetration into the choroid”, Opt Express, 13, 3252 (2013). PMID: 19495226.
- M Adhi et al., “Enhanced visualization of the choroido-scleral interface using swept-source OCT”, Am J Ophthalmol, 157, 1272 (2014). PMID: 24220884.
- E Moult et al., “Ultrahigh-speed swept-source OCT angiography in exudative AMD”, Ophthalmic Surg Lasers Imaging Retina, 45, 496 (2014). PMID: 25423628.
- A Invernizzi et al., “Comparing optical coherence tomography findings in different aetiologies of infectious necrotising retinitis”, Br J Ophthalmol, 102, 433 (2019). PMID: 28765144.
- E Jonathan et al., “Correlation mapping method for generating microcirculation morphology from optical coherence tomography (OCT) intensity images”, J Biophotonics, 4, 583 (2011). PMID: 21887769.
- L An and R Wang, “In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography”, Opt Express, 16, 11438 (2008). PMID: 18648464.
- E Novais et al., “Choroidal Neovascularization Analyzed on Ultrahigh-Speed Swept-Source Optical Coherence Tomography Angiography Compared to Spectral-Domain Optical Coherence Tomography Angiography”, Am J Ophthalmol, 164, 80 (2016). PMID: 26851725.
- L Nakayama et al., “The retinal foveal avascular zone as a systemic biomarker to evaluate inflammatory bowel disease control”, Int J Retina Vitreous, 6, 5 (2019). PMID: 31406581.
