What could you do with better imaging? With better information at your fingertips, how many more ocular diseases would be diagnosed earlier – before damage became irreversible? I hope to help you find answers to these questions with the photoacoustic remote sensing microscopy (PARS) technology developed in my lab; PARS enables structural and functional imaging of the eye – with high resolution.
Given its strengths, there is virtually no limit to the potential applications of PARS in ophthalmology. We can use it for early diagnosis of most blinding diseases and anything else that can be detected based on functional information, oxygen saturation, oxygen metabolism, and blood flow. And we can measure this information accurately down to a single capillary or – in some cases – down to a single red blood cell. This ability may extend to measuring the thickness of the RPE layer and melanin concentrations. We are also able to take advantage of the various optical absorptions of different contrast agents and drugs – thereby enabling measurement of drug concentrations in the eye, which could open a new field in pharmaceutical development.
Making waves
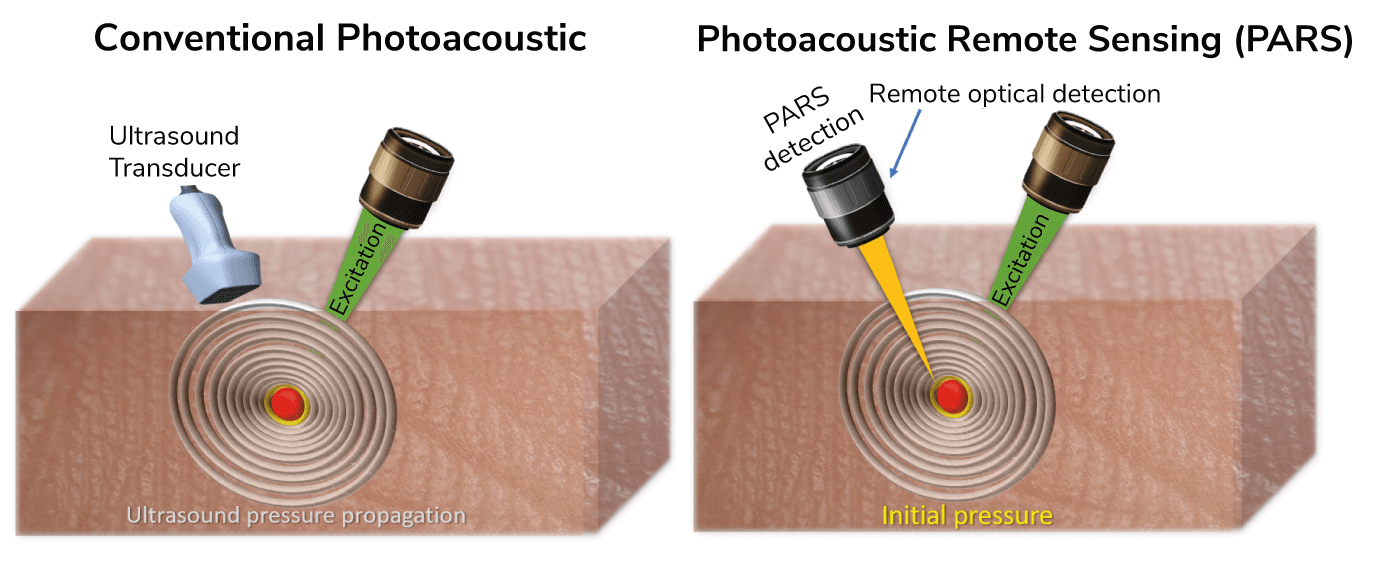
Photoacoustic imaging, in general, uses light energy to generate ultrasonic sound waves in a sample – the waves can be translated into an image – much like the ultrasound technology we’re all familiar with.
So why isn’t it already being used for eye imaging? Probably the biggest issue is the need for direct contact, with both gel and transducer touching the eye; needless to say, this is not ideal – both because of patient discomfort and the increased risk of infection from the physical contact. Furthermore, contact-based imaging directly affects the balance of vascular function and oxygen diffusion from the pressure applied to the eye, which means we are unable to study dynamic processes under conditions that are close to normality.
PARS uses an excitation laser to generate sound waves that are collected by a novel remote laser sensor that completely avoids physical contact, and collects the initial pressure right at the source. A traditional ultrasound transducer can only collect sound waves that travel to the surface of the tissue, requiring constant physical contact. To put the process in other words, when you throw a stone into a lake, shockwaves or initial pressures are generated from the moment that the stone hits the water, and these waves travel to the shore. The further you are from the place of impact, the less pressure you detect in the waves – as is the case with some transducer and traditional photoacoustic imaging. In PARS, instead of collecting the waves that have travelled to the shore, we only collect the initial pressures – the immediate pressure in the first few hundred nanoseconds after the stone (laser) hits the water (eye).
In short, PARS gives us a non-contact imaging method that, in addition to optical absorption, can provide additional imaging contrast, including scattering.
Better together
PARS is a powerful technique but, by harmonizing it with OCT, we are able to access the best of both worlds. PARS provides accurate measurements directly from optical absorption and OCT provides optical scattering, so they work in concert with each other. PARS can also provide optical scattering (and a few other light-matter interactions), but OCT is the gold standard in the field of ophthalmology – and the images are easy to understand – so we wanted to make sure that PARS images were collected from the same exact location to provide verification for what we are imaging and how we are imaging it. In future, PARS will be able to function as a stand-alone technology and for many applications OCT won’t be needed.
The imaging penetration depth is related to the wavelength and the tissue type we are looking at – which makes the eye especially advantageous. With visible wavelengths, we cannot penetrate more than two or three millimeters into scattering tissues. As the eye is transparent to most of the wavelengths we use, there is no limit to the depth we can achieve. We can look at the retina and the structures at the back of the eye easily. The RPE layer may bring a unique challenge given that the role of this tissue is to absorb light – and that may limit the ability to image the choroid and beyond. As most of the light will be absorbed by the RPE layer, not enough photons will get through to generate the photoacoustic waves that create the image; however, we are actively working on techniques to overcome this.
One of the major advantages of PARS is the ability to measure optical absorption directly and accurately. Almost everything absorbs light: RNA, DNA, melanin, lipids, hemoglobin, and more. With PARS, we can directly pick up this information – and that results in high-resolution imaging; if needed, we can see right down to a single red blood cell and even gain functional details down to the capillary level. And we can use any wavelength in the visible light range and even the infrared range to visualize what we want to see.
Right now, the opportunities seem limitless. But we need to do more investigations and run clinical studies to discover the true potential of PARS. I suspect that PARS will give access to biomarkers we’re not currently able to see – biomarkers that will become critical prognostic indicators for disease activity.

Tackling translation
We are optimistic but we are also perfectionists and realists – and we are actively working to address any (current and potential) limitations in a bid to further improve the technique. For example, we do not have fast 3D imaging capabilities – yet (though we have patented a method…). Perhaps the biggest barrier to successful implementation of PARS is the fact that it takes time to introduce a brand new technology that provides unique information into the clinic.
I think PARS will have a revolutionary impact on a number of clinical settings outside of ophthalmology. For example, we hope to have an impact in surgical oncology by providing real-time information with the aid of a surgical microscope. PARS is already being integrated into the first histology device for either in situ assessment of cancer margins during surgery or as a stand-alone tabletop device to reduce the time it takes to do histological analysis; in fact, that has been my main focus for the last two years. We can also reduce the size of the technology down to a single-mode fiber, which could be used in endoscopic applications.
I believe that, in a year or so, our first products for histology imaging should be commercially available. To get there, we’ve built a highly skilled team and developed a comprehensive plan. From a list of 50 possible applications in my office, ophthalmology has high priority!
My dream is to see PARS as a gold standard in optical imaging in many applications –especially in ophthalmology. Like any scientist, I want to see my findings making impact in our society.
Most of our work on PARS– and its translation to a clinical setting – is supported by my company illumisonics, which was established specifically to patent and commercialize PARS applications. I’ve always believed that academia and industry need to work together side by side because they complement one another so well. And now, wearing two hats, I know that to be true. The real philosophy behind my research is to deliver real-world impact – and that is exactly what I intend to do.
The Sweet Sound of Serendipity
I actually discovered the PARS method by accident during my PhD. Whilst working on a completely different project, I noticed that it was possible to collect pressure or photoacoustic signals that were generated through the air. From that point onwards, I have focused my career on further developing the physics behind PARS – and translating that knowledge into a new optical imaging modality.
When it comes to light and optical imaging methods, one natural application is ophthalmology; after all, our eyes have evolved to collect light. We were also aware that there is a continuous drive to better understand the function of the eye – and that demands increasingly sophisticated imaging modalities, which inspired to use the physics behind PARS to work on an ophthalmic device.
References
- Z Hosseinaee et al., “Functional and structural ophthalmic imaging using noncontact multimodal photoacoustic remote sensing microscopy and optical coherence tomography,”, Scientific Reports 11, 11466 (2021). PMID: 34075105.
