People have been dropping aqueous formulations into the eye for decades – and living with the consequences for just as long. The 30-50 μl drop-size associated with aqueous liquids activates the blink reflex, such that most drug is cleared from the eye within 15-30 seconds. This rapid clearing offers little opportunity for transcorneal uptake, resulting in low bioavailability (c. 1-10 percent) and limited efficacy. And the problem with water doesn’t end there: aqueous formulations need preservatives to prevent microbial growth, and often require excipients to maintain the active ingredient in solution. These additives contribute to the high rates of adverse events – and low therapy adherence – associated with standard eye-drops. When patients won’t take drugs, we have a problem.
The solution to the problem is not an aqueous one. It is called EyeSol®, and is based not on water, but on semifluorinated alkanes (SFAs). This novel carrier liquid – the first and only water-free eye-drop technology, anywhere – is transforming ocular therapeutics. Critically, its low surface tension and low viscosity permit a very low (~10 μl) droplet size, which does not activate the natural blink reflex. Residence time of up to 240 minutes (1) can be achieved for EyeSol® compared to only 15-30 seconds as known for water-based eye drops.
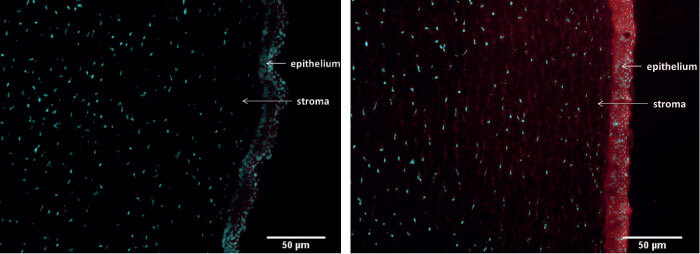
Additionally, EyeSol® spread across the cornea to a greater extent than water, such that EyeSol®-formulated drugs have access to a greater corneal and ocular surface area than aqueous formulations. These features improve drug permeation and bioavailability (Figure 1): EyeSol® formulations exhibit a far higher bioavailability of drugs in ocular tissues such as cornea compared with aqueous formulations (2).
The water-free nature of EyeSol®provides the opportunity to develop long term stable drug products of even active substances which are sensitive to hydration and oxidation (e.g. Omega 3). Furthermore, zero water means zero microbial growth, so EyeSol®requires no preservatives, which also allows EyeSol® formulations to be packaged in multi-use containers.
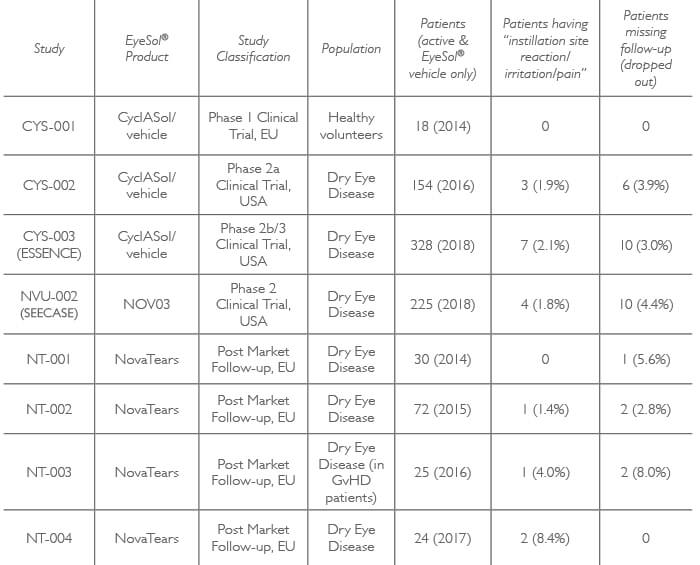
Equally importantly, the absence of preservatives contributes to EyeSol’s benign profile: SFAs which are the main ingredient of EyeSol® formulations are physiologically and chemically inert; require no irritating additives; have neither pH nor osmolarity; and form a strong and long-lasting supramolecular film that enables non-blurry vision. In effect, the eye does not even know they are there! Data from over 800 clinical trial patients – showing low adverse event and trial drop-out rates (Table 1) – confirm the high tolerability and safety of the EyeSol® technology.
One EyeSol® product is on the market outside of the USA – but that is only the start for this novel drug technology. This water-free technology is compatible with both lipophilic and hydrophilic molecules.
Lipophilic molecules dissolve directly while a novel generation of suspension formulations with unique features can be developed for hydrophilic drugs due to the amphiphilic nature of EyeSol®. Thus, EyeSol® formulations are compatible with many drug classes; examples include: prostaglandins, cannabinoids, polyunsaturated fatty acids, vitamin E derivatives, and macrolide immunosuppressants. Furthermore, Novaliq has identified a number of interesting APIs which are compatible with the EyeSol® technology. Indeed, Novaliq’s pipeline includes first-in-class products across all key ophthalmic indications, and looks set to flood (no irony intended) the market with a new generation of eye-drops. The company now invites pharmaceutical companies to benefit from the Novaliq disruptive technology and strong IP: partnership with Novaliq has the potential to enable formulation and commercialization of a wide range of drugs – from poorly soluble small molecules to proteins.
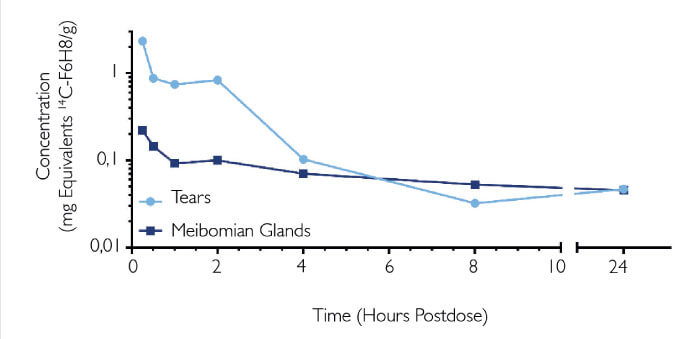
NOV03 (comprising perfluorohexyloctane) is a preservative-free ophthalmic solution and the first drug in development that targets DED associated with Meibomian gland dysfunction. It sounds counterintuitive: a water-free product for dry eye. But its unique properties enable it to quickly restore the lipid layer, thereby preventing excessive tear evaporation. Its longer-term benefits arise from penetration of the Meibomian glands (1), where it helps solubilize and distribute Meibum (Figure 3) (3, 4).
John A. Hovanesian, Specialist in Cataract, Refractive, Cornea and Pterygium Surgery at Harvard Eye Associates, treats many dry eye cases. The Ophthalmologist editor asked him
what he thought of NOV03.
Hovanesian notes that the experience of application is almost indiscernible: “The drop is so small, and distributes so smoothly across the eye, that you have almost no sense of anything going in.” But the patient feels the difference: “An immediate sense of relief – the unique properties of NOV03 tremendously enhances ocular surface stability, smoothness and comfort.” He stresses the utility of NOV03 in stabilization of Meibomian secretions. “Virtually all patients with dry eye have some degree of Meibomian dysfunction – so this product should help almost all of them.” And the future? Hovanesian is certain of the platform potential of EyeSol® : “It supports higher drug concentrations in the eyedrop, it increases duration of contact with the cornea, and it is better tolerated by the patient. You don’t need preservatives, and you don’t need to package it in expensive, single-dose containers.” So the EyeSol® products would likely be not only better, but also cheaper: Any final thoughts? “If one product is uncomfortable for up to 25 percent of patients, and the other is uncomfortable in only 2 percent, which one will you prescribe? Obviously, the one that patients will continue taking.”

For more information regarding EyeSol®, visit www.novaliq.com
References
- S Krösser et al., “Ocular and systemic distribution of 14-C perfluorohexyloctane following topical ocular administration to rabbits”, Poster, ARVO 2018. P Agarwal et al., “Semifluorinated alkane-based systems for enhanced corneal penetration of poorly soluble drugs.”, Int J Pharm, 538, 119-129 (2018). PMID: 29339249. Novaliq data on f ile. M Krafft and J R iess, “Chemistry, physical chemistry and uses of molecular fluorocarbon-hydrocarbon diblocks, triblocks and related compounds – unique ‘apolar’ components for self-assembled collid and interface engineering”, Chem Rev, 109, 1714-1792 (2009). PMID: 19296687.
