- The ALTAIR trial recently provided evidence to support new aflibercept treatment regimen
- Eight-weekly aflibercept dosing was associated with 96-week efficacy and safety outcomes similar to those of a monthly ranibizumab regime
- Later intervals varied from 8 to 16 weeks, the interval being adjusted on the basis of individual patient characteristics
- Treat-and-extend dosing earlier in the first year of aflibercept treatment has resulted in excellent visual outcomes, and reduced burden of treatment for many patients.
Physicians now have the option of treating wet AMD patients with aflibercept administrations spaced at intervals beyond every 8 weeks in the first year (1). True, a loading period (three doses spaced four weeks apart) is still required, but the potential reduction in treatment burden nevertheless is significant. How does the evidence stack up for the risk-reward balance of this new regime? The most recent addition to the weight of data supporting treat and extend (T&E) aflibercept comes from the ALTAIR trial (see sidebar, “ALTAIR: Time for T&E”), an interventional study evaluating efficacy and safety of variable interval aflibercept administration in Japanese subjects with wet AMD.
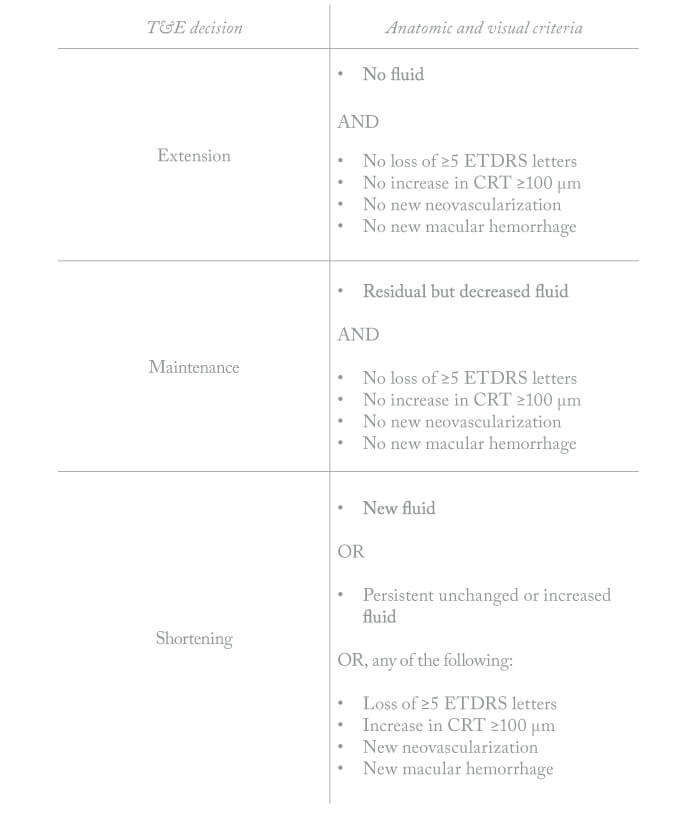
Phase III trials of aflibercept showed that an eight-weekly dosing regime of aflibercept was associated with 96-week efficacy and safety outcomes similar to those of monthly ranibizumab (2, 3). In brief, after an initial three doses spaced by intervals of a month, the ALTAIR treatment interval was extended to two months; subsequent intervals varied in the range 8–16 weeks, the interval being adjusted in two- or four-weekly increments on the basis of anatomic and visual measurements (see Table 1).
The trial results (see Table 2) indicate that both treatment arms enjoyed similar benefits with regard to visual and anatomical outcomes (4, 5, 6). Furthermore, improvements in visual acuity and retinal thickness were sustained through the 96-week follow-up, although there was a small numerical decline in BCVA letter score from week 52 in the second year (-1.4 letters in the two-week adjustment group and -2.3 letters in the four-week adjustment group). The proportions of patients who gained 15 letters or more from baseline to week 96 ranged from 28.5 percent to 31.7 percent, similar to the proportions that gained ≥15 letters from baseline at week 52 (range, 30.9 percent to 32.5 percent). Robust vision gains were achieved, with up to 60 percent of patients maintained at treatment intervals of ≥12 weeks and more than 40 percent of patients maintained at the maximum interval of 16 weeks by week 96.
What should ophthalmologists take home from this? The rapid and sustained improvements in visual acuity and central retinal thickness observed during the first year of treat-and-extend aflibercept were largely maintained during the second year – with both two- and four-week extensions. With regard to treatment burden, it is notable that injection frequency in the second year was substantially lower than that in the first 12 months of treatment. Overall, patients received an average of 10.4 injections through two years, with fewer than four injections given in the second year. Use of treat-and-extend dosing earlier in the first year of aflibercept treatment for wet AMD achieves excellent visual outcomes and, for many patients, may reduce the burden of treatment in terms of injection frequency and clinic visits.

The ALTAIR Phase IV clinical trial (NCT02305238) evaluated the efficacy and safety of two different treat-and-extend (T&E) intravitreal aflibercept dosing regimens in patients with treatment-naïve neovascular age-related macular degeneration (7). Data were collected between December 2014 and November 2017.
- Design: randomized, open-label, multicenter (40 sites in Japan)
- Study population: Japanese men and women aged ≥50 years
- Inclusion criteria: active primary subfoveal choroidal neovascularization (CNV) lesions secondary to wet AMD determined by fluorescein angiography and BCVA of 73 to 25 letters (approximately 20/40-20/320 Snellen equivalent)
- Follow-up period: 96 weeks
- Protocol:
- Monthly aflibercept doses x3 followed by a further injection two months later and retreatment at variable intervals thereafter.
- Week 16: 255 patients were randomly assigned in a 1:1 ratio to one of two different treat-and-extend dosing regimens: two-week adjustment T&E (n=124) or four-week adjustment T&E (n=123) dosing groups.
- From week 16 through to study completion, the minimum treatment interval was eight weeks and the maximum interval was capped at 16 weeks.
- Outcome measures: BCVA change from baseline; reduction in central retinal thickness
- Results:
- Safety: As per aflibercept Phase III studies
- Injection intervals: A last treatment interval of 12 weeks or beyond at final study visit at week 96 was achieved by 56.9 percent and 60.2 percent of participants in the 2-week and 4-week adjustment groups, respectively. The mean (± SD) last injection interval up to week 96 was 12.2 ± 3.6 weeks in the two-week adjustment group and 12.5 ± 3.6 weeks in the four-week adjustment group.
- T&E percentages: 42 percent of patients were extended to 16 weeks and maintained at that maximum treatment interval at week 96. In contrast, 25 percent of study participants remained on bimonthly aflibercept therapy with no extension throughout the trial.
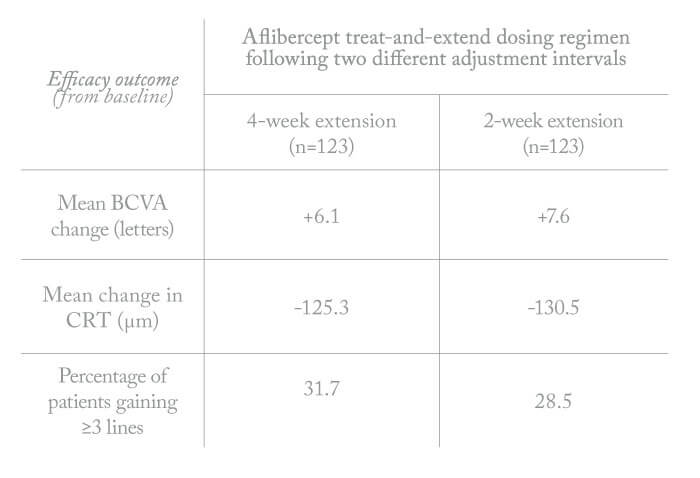
- Mean BCVA change at week 96: +7.6 letters in the 2-week adjustment group (n=123) and +6.1 letters in the four-week adjustment group (n=123)
- Mean change in central retinal thickness at week 96: ~ -130 µm in both treatment groups
- Conclusion: Improvements in BCVA and central retinal thickness achieved during the first year of T&E aflibercept treatment are largely maintained during the second year, in both two-week and four-week extension regimes.
References
- Electronic Medicines Compendium, “Eylea® summary of product characteristics”. Available at: https://bit.ly/2lKP923. Accessed: September 17, 2019.
- J Heier et al., “VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration”, Ophthalmology, 119, 2537 (2012). PMID: 23084240.
- U Schmidt-Erfurth et al., “Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies”, Ophthalmology, 121, 193 (2014). PMID: 24084500.
- M Ohji et al., “Two different treat-and-extend dosing regimens of intravitreal aflibercept in Japanese patients with wet age-related macular degeneration: 96-week results of the ALTAIR study”. Presentation at the 18th European Society of Retina Specialists (EURETINA) Congress; Vienna, Austria, September 20-23, 2018.
- M Ohji et al., “Use of intravitreal aflibercept treat-and-extend dosing for wet age-related macular degeneration: 96-week ALTAIR results”. Presentation at the American Academy of Ophthalmology 2018 Annual Meeting on 27 October 2018, Chicago, USA.
- M Ohji et al., “Two different treat and extend dosing regimens of intravitreal aflibercept for wAMD in Japanese patients: 52-week results of the ALTAIR study.” Presentation at the 17th European Society of Retina Specialists (EURETINA) Congress; Barcelona, Spain, September 7-10, 2017.
- ClinicalTrials.gov Identifier: NCT02305238, “Japanese Treat and Extend Study of Aflibercept in Neovascular Age-related Macular Degeneration (ALTAIR)”. Available at: https://clinicaltrials.gov. Accessed: September 17, 2019.
