
- Dysfunction and death of retinal cells, and consequent vision loss, can be inhibited by direct electrical stimulation of vulnerable cells in the visual pathway
- We achieve this effect not by invasive, bulky implants that generate unnatural visual stimuli, but by intravitreal injection of a colloidal suspension of nanoparticle devices that support natural vision
- These “quantum dots” diffuse into the retina where they transduce visible light energy into electricity, thereby triggering action potentials in adjacent neural cells and preserving function
- Our initial field of focus is retinitis pigmentosa; we are following encouraging Phase I results with a controlled, 15-patient clinical trial, expected to be complete by the end of 2020.
While I have been involved in ophthalmology since 1999, my career in the medical device industry started with CAT scanners as well as ultrasound machines and other devices in fields including cardiology, critical care and anesthesia. I migrated into ophthalmology in the laser therapeutic space as President of Coherent Medical, and have been in the field ever since. I’m now at a point in life where I try to spend my time only on developing new technologies that will make a significant difference to patients – opportunities that can truly change healthcare for the better. With some much recent innovation, where might we find such step-change opportunities in ophthalmology today?
The answer to that question lies partly in the shifting demographics of emerging markets (aging populations will increasingly suffer from age-related, back-of-the-eye diseases) and partly in an appreciation of the direction of research throughout the last 20 years (which, with a few worthy exceptions, has been heavily directed at refractive, front-of-the-eye conditions). Going forward, the next 20 years will see much of the research efforts focused on the retina – and that is why I am working on the ophthalmological application of nanoparticles known as quantum dots (see box: Quantum dots).
The rationale behind our approach is very simple. In many retinal diseases, cellular degeneration and vision loss involve dysfunction or death of photoreceptors and/or associated neurons in the visual pathway – but you can inhibit this process, and maintain functionality, by directly triggering activity in visual pathway neurons. We achieve this via intravitreal injection of quantum dots. It’s an entirely new approach to the retinal implant concept, and it is potentially applicable to any condition associated with retinal degeneration. So how does it work? Briefly, it’s a sophisticated take on an idea that’s been around well before Mary Shelley wrote Frankenstein – namely, electrical stimulation of neural responses.
Prior to the use of quantum dots, the electrical innovation approach relied on invasive, retinal implants: for example, the Second Sight “Argus” device. But these types of retinal prostheses have some fundamental drawbacks: the invasive nature of the implantation procedure limits their use to patients who have lost almost all vision, and the nature of the device is such that it presents the brain with a stimulus that the visual system must learn to interpret. Our approach, by contrast, involves the application of a suspension of quantum dots. The size and construction of these particles are such that they easily diffuse into and across the retina, assisted by the natural processes of the eye which move the vitreous through the retina. In effect, we are using the vitreous as a reservoir to supply quantum dots to visual neurons.
The quantum dot mechanism of action is strikingly simple. While in the retina, the nanoparticles are stimulated by visible light entering the eye – and if a quantum dot is stimulated while it is in close proximity to a neural cell, it triggers an action potential in that cell which is interpreted as vision by the brain. Thus, the effect of photovoltaically active nanoparticles diffused throughout the retina is to electrically stimulate a large range and number of neuro-retinal cells. This form of direct stimulation has been reported (1-3) to prevent cell death in the ganglion cell layer, inner nuclear layer and outer nuclear layer, the latter of which contains photoreceptor cell bodies.
In this way, administration of photovoltaic quantum dots to the retina may preserve the function and extend the lifetime of photoreceptor-dependent neurons, thereby arresting the visual decline associated with degenerative diseases of the retina. Furthermore, this effect may be achieved without the invasive surgery required for fixed, permanent implants – all that is needed to administer quantum dots is a standard intravitreal injection, just like the millions already given to AMD patients across the globe. Moreover, the injected aliquot can be very small – a volume far less than that of a sharpened pencil point holds over 12 trillion quantum dot nanoparticles!
The original work on quantum dot-mediated electrical stimulation of visual neurons was carried out by Jeff Olson at the University of Colorado. Using the Royal College of Surgeons rat model of inherited retinal degeneration, he showed that quantum dots could enhance cell survival and visual function (4). Subsequently, our preliminary, open-label clinical trial in Mexico assessed the safety of quantum dots in 20 patients with severe RP-associated vision loss. No significant treatment-related adverse events were recorded; one person had a heart attack, which was unrelated to treatment, and another had self-resolving injection-site inflammation, which is not unusual for the intravitreal administration route. But, perhaps more interestingly, 78 percent of the patients reported subjective improvements in visual function: for example, brighter and clearer vision. One patient said she’d been able to cook dinner for the first time in seven years; another reported that he’d been able to walk down the street without stepping in so many puddles. And when there was an effect, it was rapid – benefits were seen within weeks, if not days. These are anecdotal accounts, of course, and the trial was not controlled, but they are nevertheless suggestive of an encouraging quantum dot effect. It’s worth noting that these patient accounts were independent; they were not in communication with each other.

These data on the potential efficacy of quantum dots supported our recent fundraising activities. We’ve now had two phases of Series B funding: the first allowed us to address a number of questions regarding the design of a controlled clinical trial, and the second will pay for the trial itself. A key outcome from the first of these two phases was obtaining the regulator’s agreement that although an injectable quantum dot product looks like a drug, it is, in fact, a device. This is because there is nothing “pharmacological” about quantum dots – they don’t bind to or otherwise engage cells, they just provide electrical stimulation on their way through the retina. Another key point was that we were eligible for the FDA Early Feasibility Study pathway, which means we have a relatively low-cost route for early clinical studies. Basically, this pathway is the FDA’s way of encouraging companies to conduct early trials in the US rather than in other countries. Thirdly, the FDA agreed with our proposal to use functional vision – navigation of a mobility course – as a clinical end-point rather than solely relying on visual acuity and other similar standard endpoints. Our mobility course was developed by Ora and is conceptually similar to the one used by Spark Therapeutics, which resulted in the company getting clearance for its product. The ORA mobility test is a sophisticated design: it can be configured to control lighting in a variety of different physical locations, enabling efficient multi-center clinical trial use. Ora-VNC mobility courses accommodate various types of vision loss as well as different severities, and can be customized with regard to parameters such as color, contrast, and obstacles.
The second tranche of our Series B will support the company as we move forward with our clinical program, the US-based Early Feasibility Study (5). We intend to engage three sites; at present, we are confirming the arrangements, and carrying out the final phases of preclinical work – demonstrating that the product batch is non-toxic, for example. Getting this work completed will be the last hurdle to jump through before being granted investigational device exemption by the FDA, which we must have prior to initiating our controlled, 15-patient clinical trial in early 2020. We expect to have the final data by the end of 2020. Overall, the clinical development will be relatively straightforward – one byproduct of addressing an unmet need where there are no other therapies makes our trial far less complex.
At this point, we are entirely focused on retinitis pigmentosa (RP) – it is a highly unserved market and RP patients desperately need effective therapeutic options. Even if you include development-phase RP products, the picture is daunting: virtually all efforts are focused on gene therapy or stem cell therapies. When you consider that there are more than 40 gene defects associated with RP, and that each defect could require development of a separate gene or cell therapy, it is clear that these approaches are unlikely to provide a near-term alternatives for most RP patients. Our hope is that the quantum dot approach will offer a substantive and reasonably near-term therapeutic choice.
The simplicity of our approach also provides significant advantages compared to other alternatives, namely that the treatment is injected as opposed to undergoing invasive surgery. With that said, the quantum dot RP therapy will not be a single-shot cure – we would expect the treatment to be repeated, perhaps three to four times a year, somewhat less than anti-VEGF therapy for AMD. However, there are benefits to the temporary nature of the quantum dot effect: you don’t have to explant anything if it doesn’t work; if something better comes along, you can just stop treatment; and if something complementary is developed, there is the possibility of additive or synergistic therapeutic effects. We believe we are on the road to providing a meaningful option to a population of patients who have been underserved for far too long.
- Nanoscale-sized, biocompatible semiconductor crystals
- Exhibit photovoltaic response – conversion of light into electricity – when illuminated by particular wavelengths
- Optoelectronic properties change as a function of size, shape, material and coating: hence, Quantum Dots can be designed such that they are excited only by particular wavelengths in the visible light range
- Voltaic response therefore can be fine-tuned to correspond to light of strictly defined wavelength
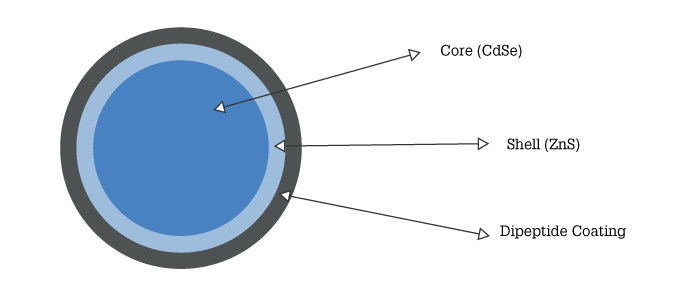
- Quantum Dot technology developed by 2C Tech (Irvine, USA) is based on a cadmium-selenium crystalline core encapsulated in a zinc sulphide shell, which is itself coated by a hydrophilic dipeptide
- This design supports formulation as a colloidal solution, protects the metallic elements from oxidative damage, and enhances efficiency of photovoltaic response
- In vivo injection is followed by diffusion and eventual elimination by normal biological processes
References
- M Watanabe and Y Fukuda, “Survival and axonal regeneration of retinal ganglion cells in adult cats”, Prog Retin Eye Res, 21, 529 (2002) PMID: 12433376.
- H Schmid et al., “Neuroprotective effect of transretinal electrical stimulation on neurons in the inner nuclear layer of the degenerated retina”, Brain Res Bull, 79, 15 (2009). PMID: 19150490.
- M Pardue et al., “Neuroprotective effect of subretinal implants in the RCS rat”, Invest Ophthalmol Vis Sci, 46, 674 (2005). PMID: 15671299.
- J Olson et al., “Intravitreal silicon-based quantum dots as neuroprotective factors in a model of retinal photoreceptor degeneration”, Invest Ophthalmol Vis Sci, 53, 5713 (2012). PMID: 22761263.
- Globenewswire, “First Clinical Program Initiated Using Nanoparticles to Treat Retinitis Pigmentosa” (2019). Available at: https://bit.ly/2CMV63a. Accessed November 11, 2019.
