
- Replacing the vitreous with silicone oil can reduce scar tissue formation in vitreoretinopathy and diabetic retinopathy, but attempts to combine the oil with pharmaceuticals have not yet been successful
- A multidisciplinary team at the University of Liverpool, UK, is working on achieving controlled drug release from silicone tamponades to prevent retinal scarring
- Milestones of the research so far include choosing the right model drug and determining its solubility in oil, as well as modulating the release kinetics of oil-drug combinations
- The team has proven that modified silicone oils are non-toxic to relevant cells, they enhance drug solubility and extend drug release.
Conditions such as proliferative vitreoretinopathy and proliferative diabetic retinopathy can lead to retinal detachment (RD), followed by sight-threatening scarring. Silicone oil tamponades – replacement of the vitreous with silicone oil – reduce the probability of scar tissue formation, but the success rate of this approach has remained static for years. There are two reasons why efforts to combine silicone oil tamponades with pharmacological anti-scarring approaches have had little success.
First, many drugs are designed to be hydrophilic, and therefore are substantially oil-insoluble. Paradoxically, this forces us to use lower than optimal drug doses, because the drug collects around the surface of the oil, resulting in an excessively high local accumulation. Second, even lipophilic drugs are associated with imperfect pharmacokinetics in these tamponade systems: the oil releases the compound into the eye too quickly, resulting in rapid drug clearance and poor efficacy. In consequence, there are at present no accepted oil-antifibrotic combinations.
Addressing this situation requires a multidisciplinary approach; fortunately, multidisciplinary interactions are the norm in the Department of Eye and Vision Science at the University of Liverpool, UK. In particular, our non-clinical, academic researchers collaborate closely with clinical teams at St Paul’s Eye Unit; the advantages of this system are reflected in our history of translating laboratory research, via industrial collaborations, to the bedside. This environment has allowed me to apply my biomaterials expertise to the development of new ways to reduce retinal scarring.
My team, including Steve Rannard and Tom McDonald, and researchers Maude Le Hellaye and Helen Cauldbeck is made up of chemists, biologists, bioengineers and surgeons, working with an industrial partner. We are focused on achieving sustained, controlled drug release from silicone oil tamponades, with the aim of developing a tamponade that will truly prevent retinal scarring (see: “Why focus on drug delivery from silicone oil tamponades?”). And now, after seven years’ hard work – and the wonderful support from Fight for Sight and EPSRC – we are approaching the point where our system can benefit patients.
- We know that drug delivery from silicone oils is, in principle, possible and safe, both in animals and people: for example, aspirin (1) and triamcinolone (2, 3)
- Problems with current approaches include:
-
- poor drug solubility in oil (leading to ineffective drug loading, precipitation, requirements for separate devices or additional drug injections, and drug accumulation at oil-eye interface)
-
- release of the drug from the oil too quickly such that it is rapidly cleared from the eye, resulting in poor efficacy
- By improving the drug delivery characteristics of oil-drug combinations, we can avoid the aforementioned problems and improve the efficacy and predictability of tamponade-mediated scarring inhibition.
Our key task was to modify the drug substance or oil – or both – so as to achieve a drug-release profile that endures over the several weeks that RD patients are at risk of scarring. And that required the successful execution of multiple smaller tasks.
First, we had to pick a relevant model drug to use in our studies. We chose all-trans retinoic acid (atRA) for the following reasons: it is lipophilic; it has a role in eye physiology; it has diverse actions suggestive of an anti-scarring effect (anti-proliferative; anti-oxidative; reduces matrix production; maintains epithelial phenotype; antagonizes RPE65) and, importantly, it is the subject of published work (4) against which we can benchmark our own investigations.
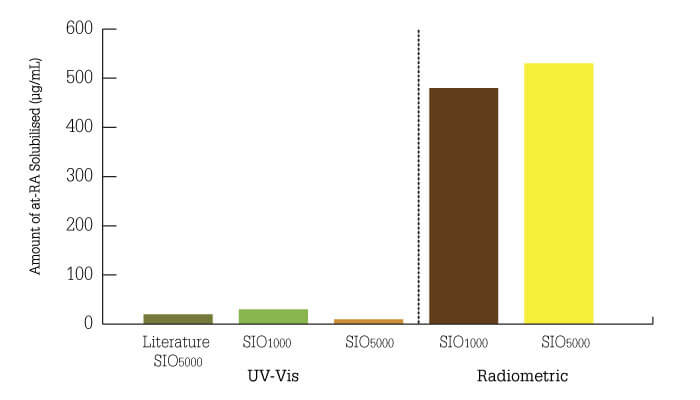
The next task was to determine the solubility of atRA in silicone oil, which turned out to be a surprisingly complex exercise. Our initial data, using high-performance liquid chromatography (HPLC) with UV detection, suggested solubility levels 25 percent higher than published estimates (4). There are, however, problems when applying UV-visible spectroscopy to the analysis of biological fluids, such as vitreous humor and cell culture media – not least, the overlap in absorption peaks of media components and drugs. The challenges forced us to develop a radiochemistry method for measuring drug solubility in oil, which led us to one of our biggest surprises (5) – atRA solubility in silicone oil is actually over twenty times higher than reported! (Figure 1)
Figure 1. Development of a novel radiometric analysis method reveals (5) that the solubility of all trans retinoic acid is 20-fold higher than previously thought (4).
Once we were confident that we could accurately measure the oil solubility of drugs, we turned our attention to modulating the release kinetics of oil-drug combinations. To this end, we developed two novel technologies, comprising novel polymers and polymer-drug conjugates
We synthesized (6) a range of novel graft copolymers containing dimethylsiloxane and ethylene glycol repeat units within the copolymer side-chains. Importantly, the dimethylsiloxane units permit solubilization in oil, whereas the ethylene glycol units provide hydrogen bond acceptor sites to promote interaction with acidic drug molecules. We demonstrated that the copolymers were non-toxic to retinal pigment epithelial cells; subsequently, radiochemistry experiments with atRA showed extended drug release from silicone oil (6). Specifically, adding graft copolymer to oil (10 percent v/v) extended drug release (for example, ibuprofen release was extended from >3 days to >9 days). However, drug release is still relatively rapid with this formulation (for example 80 percent of atRA has been released after 25 days).
The second technology (5) involved chain-end modification of polydimethylsiloxane with atRA to produce polydimethylsiloxane retinoate (PDMS-atRA). Adding this to silicone oil was shown to both increase atRA solubility and, more significantly, extend the duration of drug release. Interestingly, the drug release period was dependent on PDMS-atRA concentration, and independent of initial atRA concentration. With this technology, cumulative drug release does not reach 80 percent until ~day 50 (10 percent blend of polymer in oil) – as compared with day 16 for oil alone (Table 1). Again, the duration of release is independent of initial atRA concentration in the oil.
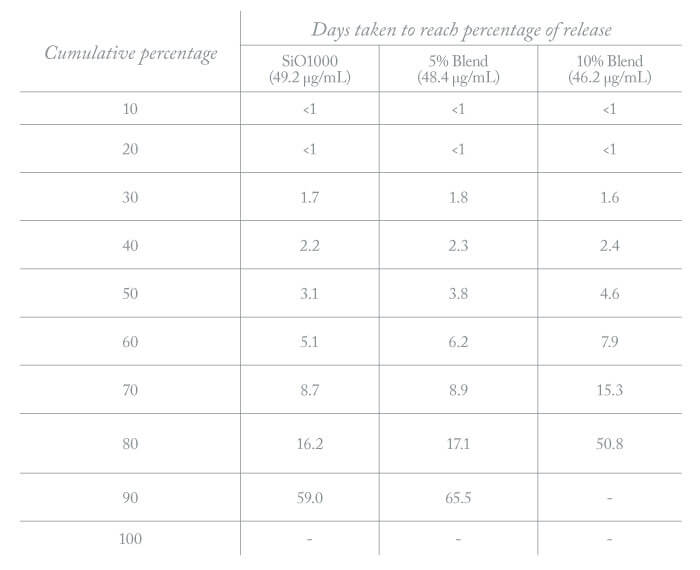
Table 1. Drug release profile from tamponade oil blended with polydimethylsiloxane retinoate (PDMS-atRA). Note that 80 percent of drug has been released by day 16 with silicone oil as compared to day 50 with the silicone oil-polymer blend.
We have shown that our modified silicone oils are non-toxic to relevant cell lines, and both enhance drug solubility in oil and extend drug release. Furthermore, they are stable at room temperature over extended periods, and can be sterilized using commercial protocols – both of which increase their potential for commercial development. Finally, our technology is compatible with different drugs, and can accommodate a wide range of initial drug loads – all suggestive of applicability to a broad range of drugs and diseases.
This project hasn’t always been easy, and we’ve encountered a few surprises and challenges along the way. From a practical point of view, oils can make containers pretty slippery – you have to be very careful when handling them! But now that we’ve developed the basic framework of a new ocular drug delivery system, we’re looking forward to applying it in the real world. We are already working with an industrial partner to combine our drug release technology with marketed oil tamponades; a key task is to ensure that we maintain the desirable physical properties of these products, such as easy injection and resistance to emulsification, and to ascertain that the new products are at least as effective as the existing tamponades. In brief, we believe we have developed a new paradigm in the prevention of scarring subsequent to retinal detachment, and aim to have a product ready for first-in-human studies within five years.

References
- MT Kralinger et al., “Safety and feasibility of a novel intravitreal tamponade using a silicone oil/acetyl-salicylic acid suspension for proliferative vitreoretinopathy: first results of the Austrian Clinical Multicenter Study”, Graefes Arch Clin Exp Ophthalmol, 248, 1193 (2010). PMID: 20424852.
- G Fernandes-Cunha et al., “Determination of triamcinolone acetonide in silicone oil and aqueous humour of vitrectomised rabbits’ eyes: application for a pharmacokinetic study with intravitreal triamcinolone acetonide injections”, J Pharm Biomed Anal, 89, 24 (2014). PMID: 24252721.
- M Da et al., “Distribution of triamcinolone acetonide after intravitreal injection into silicone oil-filled eye”, Biomed Res Int, 2016, 5485467 doi: 10.1155/2016/5485467 (2016). PMID: 27493959.
- J Araiz et al., “Antiproliferative effect of retinoic acid in intravitreous silicone oil in an animal model of proliferative vitreoretinopathy”, Invest Ophthalmol Vis Sci, 34, 522 (1993). PMID: 8449673.
- H Cauldbeck et al., “Modulated release from implantable ocular silicone oil tamponade drug reservoirs”, J Polym Sci A Polym Chem, 56, 938 (2018). PMID: 29610546.
- H Cauldbeck et al., “Controlling drug release from non-aqueous environments: moderating delivery from ocular silicone oil drug reservoirs to combat proliferative vitreoretinopathy”, J Control Rel, 244, 41 (2016). PMID: 27845192.
