At a Glance
- Posterior capsule opacification (PCO) occurs as a result of natural post-surgical wound healing in the eye, and can lead to patients losing some of their vision months or years after undergoing cataract surgery
- The new in vitro capsular bag model, developed by University of East Anglia scientists and West Norwich Hospital ophthalmologists, benefits from spatial organization and cell types found in real-life patients
- Researchers are working on improving the human model, replicating regenerative features of PCO and examining a range of IOLs to determine the best outcomes for patients.
At nearly 30 million operations per annum, cataract removal and intra-ocular lens (IOL) implantation is the most common surgical procedure in the world. There are good reasons for this: cataract surgery is phenomenally effective at restoring sight to patients. But imagine how frustrating it would be for an IOL recipient to find their sight disappearing all over again. Unfortunately, this is precisely what happens to those patients who experience posterior capsule opacification (PCO). After two or three years, their decline in vision is such that they need yet another procedure – laser-removal of light scattering areas. This is not only inconvenient, but also associated with a degree of risk. Obviously, patients and surgeons alike want to avoid this situation.
Developing rational approaches to inhibit or avoid PCO, however, requires some understanding of the processes at work. What causes PCO? In brief, it is the consequence of a natural wound-healing mechanism in the eye, which itself is a response to the trauma of cataract surgery. A key aspect of post-surgical wound-healing in the eye is stimulation of lens epithelial cells to proliferation and migrate. Some of these cells invade previously cell-free areas of the lens capsular bag and can grow over the IOL, which interferes with the passage of light to the retina. Consequently, many patients start to lose their vision within months or years of having cataract surgery. We know that much about PCO – but we still have a lot of questions to answer. What molecular pathways are involved, and how might we modulate them? Which IOLs are inherently less susceptible to PCO and why – and can we build on this to design IOLs that can better prevent PCO?
Model answers
We developed our in vitro capsular bag model with the above questions in mind, and with the over-arching objective of making a difference to clinical practice and patient outcomes. And to have the best chance of making a real difference, our view was that the model should reflect the human situation as accurately as possible. Other PCO models exist, from cell cultures to whole animals, but ours is the only fully human capsular bag model, and we believe it is the system most likely to be predictive of events in real patients.
It all started with a collaboration, initiated in the mid-90s, between University of East Anglia scientists and two ophthalmologists – Christopher Liu and Peter Davies – at the West Norwich Hospital. Essentially, we began by performing bench-top cataract surgery on human donor eyes. This meant that our system benefited from the same spatial organization and cell types that you find in real patients – and that seemed like a logical approach to the study of human PCO. Since then, we have modified the basic system in various ways and for different purposes. In particular, we have experimented with a range of culture conditions – minimal media, sustained high levels of supplements, timed addition of specific activators – to investigate the role of specific molecular components and pathways, which is an excellent way of teasing out the key factors that drive PCO in patients. We’ve also improved the way the artificial lens is mounted – in the latest model, the IOL is suspended – and we have made the whole system more reflective of real life by using human serum and human growth factors in the culture medium. We’ve always aimed to mimic post-surgical inflammatory events as realistically as possible, and we believe the latest version of our model (1; Box) goes a long way to achieving that.
Another advantage of our system is that we work with two matched eyes per experiment – that is, both eyes from a given donor are used, each receiving a different IOL. This dual approach enables us to compare the influence of different IOLs on PCO within a given donor, thus removing inter-donor variability from the system, which gives us much more confidence in predicting which IOLs are most likely to resist PCO in real patients.
Moving on
We’re continuing to improve the model; for example, by mimicking longer-term changes associated with PCO following cataract surgery. In particular, we want to replicate regenerative features of PCO, such as the three-dimensional structures known as Elschnig’s Pearls and Soemmerring’s Rings. Both are known to cause light scatter and impaired vision; to prevent their development, we really need a model that allows us to better understand the etiology and test preventive mechanisms. To that end, we are working on ways to replicate these events within the time window permitted by our model. We also intend to examine a broad range of IOLs: different manufacturers, different shapes, different materials. The idea is to identify modifications that correlate with PCO resistance and which therefore should result in better outcomes for patients. It’s very gratifying that we have received funding, from HOYA Surgical Optics and the Humane Research Trust, to develop our model – I am convinced that a system based on human tissues and growth factors is the best way to model human PCO.
Modeling a better future
It’s not perfect of course – there’s not an endless supply of cadaver eyes, so we don’t always have as much material as we would like. But we’re very grateful for every donation: each one allows us to move things forward a little, and each iteration of the model is an incremental step forward – an evolution that makes it better and more clinically relevant. And that’s the point; we’re trying to make a difference at the clinical level. Today, we are identifying marketed IOLs that are less susceptible to PCO, but tomorrow we will be helping develop next-generation IOLs with advanced resistance to a range of unwanted post-surgical sequelae. Time will tell!
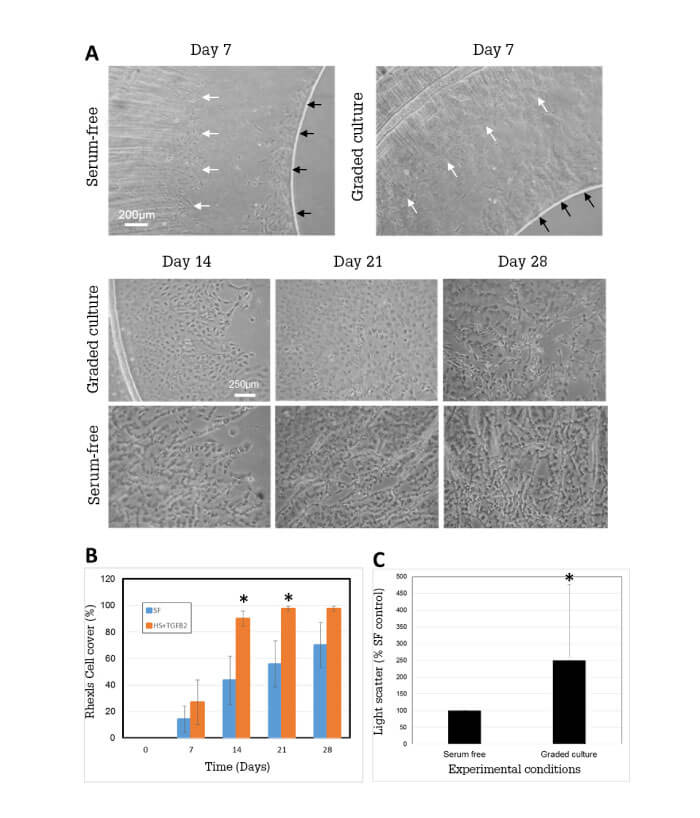
How can we most accurately represent the environment that gives rise to PCO? One way is to use human capsular bags implanted with IOLs in vitro, and to maintain them in a way that reflects the normal anatomical relationship of IOL and capsule. Such a model provides a very close representation of the clinical situation with regard to physical and cellular parameters. To reflect post-surgical reality even more closely, we can provide this model with an environment that changes over time: the initial pro-inflammatory culture medium is gradually replaced with minimal, non-activating medium.
The model
- Capsulorhexis and lens extraction performed on human donor eye to generate capsular bag attached to the ciliary body by the zonules
- Ciliary body pinned to silicone ring, such that bag containing IOL is suspended by zonules over ring lumen (thus enhancing IOL-capsule interaction)
- Preparation maintained in experimental culture medium for 28 days
- Experimental medium comprised:
- (i) serum-free medium throughout; or
- (ii) graded culture regime in which initially high levels of human serum and TGFbeta decline over time (medium is serum-free by day 15)
- End-point measurements include cell coverage, matrix contraction, matrix deposition, light scatter and myofibroblast expression
Our theory is that this graded culture regime should better mimic the post-surgical environment, by providing an initial protein-rich, stimulatory environment that subsequently declines to a non-activating environment. It is known that a number of growth factors, such as FGF, HGF and VEGF can promote wound healing, leading to PCO following cataract surgery. Many of these factors become elevated in the eye following a breakdown of the blood aqueous barrier, and thus addition of serum to our cultures mimics this process. TGFβ2 elevation following surgery is fundamentally a local event, so TGFβ2 is specifically added for this reason. Graded culture enhances growth, increases myofibroblast expression and promotes matrix contraction and matrix deposition relative to serum-free culture (Figure 1).
Our graded culture model therefore seems to reflect the PCO-favoring environment of the post-surgical eye. Our next step was to use the model to assess marketed lenses. What can the model tell us about the inherent ability of IOLs to influence PCO progression?
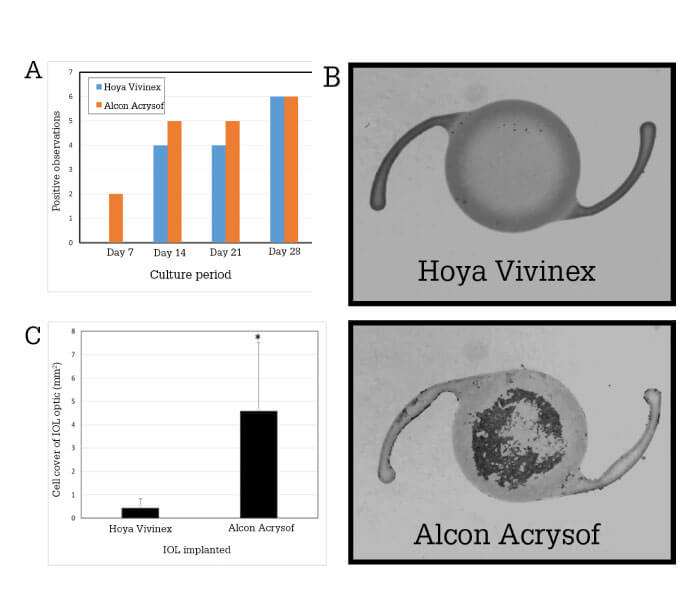
IOL comparison
- Alcon Acrysof or Hoya Vivinex IOLs were implanted in matched capsular bags (derived from the same donor)
- Matched preparations were maintained under the graded culture regime for 28 days
- Outcomes were compared with regard to: cell coverage on the posterior capsule, coverage of the IOL and light scatter within the visual axis.
The results? Vivinex IOL was more resistant to PCO (as represented by the outcome measures) than Acrysof. Specifically, although both lenses had equivalent cell coverage by day 28, cell coverage with a Vivinex implanted occurred at a slower rate and resulted in lower levels of light scatter. Moreover, cell cover of the IOL surface was less pronounced with a Vivinex IOL than Acrysof (Figure 2). These results add weight to previous observations that IOL choice may have an impact on PCO.
In conclusion, our in vitro capsular bag / graded culture regime provides investigators with an advanced PCO model that mimics the dynamic inflammatory environment of the post-surgical eye. Furthermore, it can identify differences between IOLs with regard to susceptibility to surrogate measures of PCO, and therefore serves as an excellent system to evaluate and develop IOLs, which will limit this costly and frustrating phenomenon.