
The path of technological advancement is not always driven by what is “best,” but rather by what is easier to implement and economically more profitable. Seldom do these two goals coincide. When coagulative retinal surgery moved from the original Gas-tube laser with Argon 514 nm to frequency doubled Nd-YAG lasers with a wavelength of 532 nm, it was not for therapeutic benefits, but for practical reasons. Now, to complete the circle, the shift back to 514 nm is possible with the most technologically advanced system – one that provides better patient-comfort with the time-tested benefits of the first 514 nm argon laser systems of ophthalmology.
Evolution
For simplicity, the evolution of coagulative retinal surgery will be limited to systems offered by a single company, A.R.C. Laser GmbH (Nuremberg, Germany). Their first 514 nm laser system was an Argon Gas laser, which had an overall dimension of 96 x 46 x 62cm and required an external water-cooling system and dual power supplies. Such systems entered the ophthalmic laser market in the 1970s. Three pulse spot sizes (0.25, 0.5 and 1 mm) could be achieved by focusing through the slit-lamp. Next came a 532 nm system (frequency doubled Nd:YAG laser) that was 47 x 18 x 27 cm with a small control panel. This was not because the latter was safer or more efficient, but because of the size, which meant that a separate room for the water-cooling of the laser system was no longer required. These systems were introduced in the 1990s. This shift was met with an increase in patients reporting greater pain during treatment, often requiring the entire treatment to be divided into two or more sessions.
Despite the widespread use of the 532 nm system, scientific literature was skewed in favor of the 514 nm systems for the treatment of a wider range of retinal pathologies (1). Furthermore, although the results achieved during short-term follow-ups for both systems were similar (2), one study evaluated a 10-year follow-up period for only the 514 nm laser system and found it to be safe and effective for 96 percent of patients (3).
Currently, the newest option is the CLASSIC 514 retinal photocoagulation laser system. Based on argon-microchip technology, it permits pulses in the range of nanoseconds to milliseconds. This system can be run off a rechargeable battery and has an overall dimension of 25 x 17 x 22 cm. In my experience, the new system provides the same level of patient satisfaction as the original 514 nm laser systems in terms of decreased pain and longer therapy sessions, with greater collaboration, yielding comparable treatment results.
The science of pain
But why do patients report feeling more pain with 532 nm lasers?
The retina does not possess nociceptive receptors, except for the thermoreceptors found especially around the entry of the long ciliary nerves (non-specialized nerve endings located near blood vessels). They are generally divided into low- and high-threshold receptors conducted mostly via slowly conducting unmyelinated C fibers. Low-threshold receptors provide non-painful perception at temperatures below 45°C, while high-threshold receptors give a perception of pain at temperatures higher than 45°C. Although there is a variable individual component to what temperature is perceived as painful, temperatures above 45°C are generally perceived as such (4).
Although the two laser systems differ slightly in wavelength, their absorption spectra for the principal components of the retina are different (see Figure 2). When delivering the same amount of laser energy with the two different wavelengths to the pigmented retinal epithelium, 45 percent more 514 nm energy is absorbed by the melanin, while 40 percent more 532 nm energy is absorbed by the oxygenated hemoglobin and a negligible difference for deoxygenated hemoglobin. Given that the distribution of nerve fibers usually mimics that of blood vessels (5), it is reasonable to hypothesize that the greater energy absorption with 532 nm of faster moving blood would result in a greater nociceptive stimulation, and thus a greater sensation of pain.
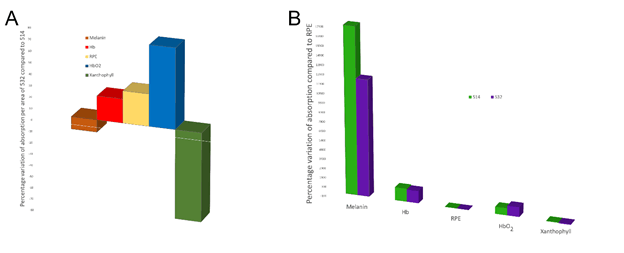
This hypothesis is especially important when considering that the energy accumulation required to reach the threshold for coagulation is not instantaneous. For example, using a pulse duration of 100 ms would result in the movement of the oxygenated hemoglobin of between 25 and 100 μm – well outside the spot generated by the laser. The validity of this hypothesis is supported by Jain and colleagues (6), who report that the volume of the spot size does not increase in proportion with laser power or pulse duration. Further studies are required to confirm this hypothesis.
The lesser of evils
In summary, the novel argon‐microchip technology (514 nm) laser system for retinal photocoagulation has the same advantages as the older, clunkier 514 nm systems but with a significant reduction in size and on battery power! Although the short-term results should be similar to those obtained with 532 nm systems, the reduced pain inflicted on patients during treatment increases the probability of completing the entire treatment in one session – increasingly important in a post-COVID world. Also, the softer generation of spots during pulses increases operator-control of spot size, permitting greater customization of therapy of both size and positioning for individual patients.
References
- The Diabetic Retinopathy Study Research Group, “Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of Diabetic Retinopathy Study (DRS) findings”, Ophthalmology, 88, 583 (1981). PMID: 7196564.
- F Bandello et a., “Double-frequency Nd:YAG laser vs. argon-green laser in the treatment of proliferative diabetic retinopathy: randomized study with long-term follow-up”, Lasers Surg Med, 19, 173 (1996). PMID: 8887920.
- M Romem et al., “Long-term follow-up of photocoagulated retinal breaks”, Br J Ophthalmol, 62, 240 (1978). PMID: 646983.
- Y Sharav et al., “Orofacial Pain and Headache” (2015). Available at: https://bit.ly/2ZOhvIS.
- P Hogg et al., “Blood vessels and nerves: together or not?”, Lancet, 360, 1714 (2002). PMID: 12480422.
- A Jain et al., “Effect of pulse duration on size and character of the lesion in retinal photocoagulation”, Arch Ophthalmol, 126, 78 (2008). PMID: 18195222.
- MA Mainster, “Wavelength selection in macular photocoagulation. Tissue optics, thermal effects, and laser systems” Ophthalmology, 93, 952-8 (1986). PMID: 21265262.
- JHJ Lock and KCS Fong, “An update on retinal laser therapy” Clin Exp Optom, 94, 46 (2011). PMID 21039846.
