
- Methods used for retinal disease monitoring should be easy to use and cost effective; currently, OCT is most often used to analyze the retinal structure
- Flavoprotein fluorescence technology promises to effectively measure the number of dysfunctional mitochondria in the retina – a biomarker that can precede structural damage
- Recent studies have confirmed the sensitivity of FPF as an indicator of retinal health and a predictor of therapeutic efficacy
- OcuMet Beacon – a system capable of assessing retinal mitochondrial function – is now moving towards commercialization.
At present, structural analysis of the retina may only identify pathological change after irreversible progression, while functional assessments can be subjective, expensive and difficult to administer. Recent developments in flavoprotein fluorescence (FPF) detection technology, however, are radically changing the status quo.
Diseases causing progressive and permanent damage to the retina should ideally be monitored with methods that identify compromised tissue while it may still be rescued. At the same time, these techniques should be cost effective and convenient to use. Unfortunately, we have been far from this ideal for many years.
Structural analysis of the retina frequently relies on OCT to measure anatomical signals, such as central macular thickness (CMT), which often correlate poorly with changes in visual acuity – and usually indicate some degree of irreversible damage if retinal thinning is seen. And functional assessments, other than visual acuity, tend to suffer from some combination of high cost, subjectivity, inconvenience for the patient and higher difficulty for the operator to perform. We need cost-effective and simple assessments which predict pathology, rather than merely following it.
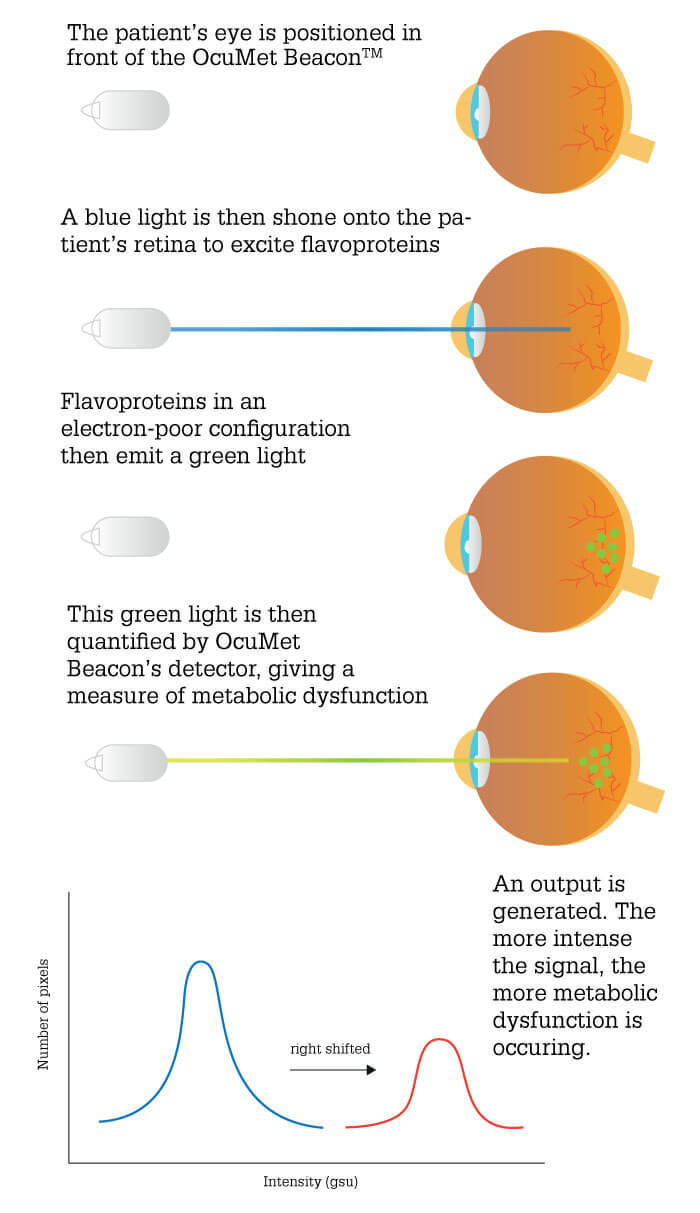
FPF levels positively correlate with mitochondrial dysfunction: the greater the number of dysfunctional mitochondria in the retina, the higher the FPF signal. Since mitochondrial dysfunction represents a significant problem for the retina – photoreceptor cells require much energy for visual cycle and clean-up processes – quantification of FPF provides a measure of retinal health (1).
This is the rationale behind our development of the OcuMet Beacon (see Box), a system that can assess retinal mitochondrial function and thereby guide patient management. We have been generating data with OcuMet Beacon since 2015; our most recent studies provide significant validation for our approach.
Impaired cellular metabolism precedes the irreversible cell loss associated with retinal disease, and signifies a reduction in the metabolic energy that retinal cells require to maintain the processes of life.
The associated mitochondrial dysfunction leads to generation of reactive oxygen species; these in turn convert mitochondrial flavoproteins from a reduced to an oxidized state. Oxidized flavoproteins exhibit characteristic, quantifiable fluorescence when excited by blue light – an ideal biomarker.
See the Beacon in operation here.
These new publications include two seminal studies (see sidebars). In the first study (2), we looked at whether we could use FPF to distinguish glaucoma suspects with ocular hypertension (OHT) from control patients, and to discriminate between mild-to-moderate versus severe primary open angle glaucoma (POAG).
We found that FPF was elevated in OHT eyes; given that these eyes have no clinical evidence of glaucomatous change, this finding strongly suggests that disease-associated macular dysfunction is detectable prior to the disease progressing to the structural changes on which most current diagnosis/monitoring procedures rely. It may also suggest that FPF could turn out to be a more useful indicator of POAG risk than IOP elevation, which does not always correlate with glaucoma development as approximately 50 percent of patients can present as normo-tensive.
In POAG eyes with advancing disease, however, it seemed at first that FPF values were not higher than those of control eyes. This is likely due to POAG-mediated retinal cell loss resulting in lower numbers of mitochondria in the field of illumination. Therefore, we adjusted for retinal cell loss by using the ratio of FPF to retinal thickness as the comparator. And that revealed a significant difference between controls and POAG eyes.
We also found that FPF is manifest asymmetrically in POAG eyes, but not in controls; this may reflect the known progression of POAG and a signature of the disease. Similarly, we found a correlation between increasing FPF and age, which may indicate that mitochondrial dysfunction contributes to the age-related increase in POAG risk.
Our second study (3) focused on patients receiving anti-VEGF treatment for centrally involved diabetic macular edema (DME); in particular, we assessed the correlation between FPF scores and visual acuity. Results showed that FPF reduction is strongly correlated with visual acuity improvement in these patients. By contrast, the relationship between visual acuity and reductions in OCT measures of anatomical pathology – CMT, retinal fluid – is much weaker. This suggests that FPF measurement can – by detecting improvements in metabolic function that precede anatomical improvements – provide earlier identification of anti-VEGF responders versus non-responders.
In combination, these papers clearly demonstrate the sensitivity of FPF as an indicator of retinal health and a predictor of therapeutic efficacy, and suggest that our metabolic marker FPF may have a role in guiding the management of patients with retinal disease.
In parallel with these studies, we have improved the OcuMet Beacon – it’s a different instrument to the one we were using in 2015. Back then, we relied on a conventional fundus camera that required a skilled operator, typically a clinician or skilled photographer; today, the Beacon is highly automated – the user needs to only tap the tablet to specify the retinal location of the desired images.
The instrument and software do everything else – zoom, focus, image capture and processing. The system is much simpler, much faster, much easier to use and more affordable. In short, it is packaged and ready to move to commercialization.
The ease-of-use aspect enables increasingly broader use of OcuMet Beacon both in optometry/ophthalmology and in applications outside this field – for example, retinal cancer, assessment of Plaquenil toxicity in arthritis patients, and monitoring of gestational diabetic women. And now that we have developed an instrument for animal use, we’re increasingly seeing FPF being used in pre-clinical drug development studies, to give an early sign of metabolic effect or possibly toxicity.
Drug development professionals appreciate the power of being able to apply the same biomarker to both human and animal studies – it helps if they can use the same measure all the way from in vitro to pre-clinical to clinical trials. We expect publication of an increasing number of studies using OcuMet Beacon over the next couple of years.
In summary, it is clear that FPF is a validated biomarker of retinal dysfunction; it can identify OHT and POAG eyes, distinguish between different stages of POAG, and provide information on anti-VEGF efficacy in DME eyes more reliably and earlier than standard assessments of retinal pathology. Furthermore, it beautifully complements other imaging modalities, as it is rapid, quantitative and non-invasive (it can be performed as frequently as you like), and does not require the application of dyes or similar agents. Everyone we have discussed the system with is very excited by its potential, and we look forward to seeing the results they will generate.
Observational, cross-sectional study (Mount Sinai, New York)
- Eyes: POAG (n=38); OHT (n=16); control (n=32)
- Methods:
- FPF measurement: OcuMet Beacon (OcuSciences Inc, Ann Arbor, USA)
- CMT measurement: Spectralis OCT (Heidelberg, Germany)
- Statistical treatment: macular FPF and ratio of macular FPF to retinal thickness was compared among the three groups with an age-adjusted linear regression model
- Results:
- OHT: both FPF (p<0.05) and FPF/CMT ratio (p<0.01) were significantly elevated
- POAG: FPF was correlated with age and FPF/CMT ratio was significantly elevated (p<0.001)
Observational, cross-sectional study (Mount Sinai, New York)
- Eyes: POAG (n=38); OHT (n=16); control (n=32)
- Methods:
- FPF measurement: OcuMet Beacon (OcuSciences Inc, Ann Arbor, USA)
- CMT measurement: Spectralis OCT (Heidelberg, Germany)
- Statistical treatment: macular FPF and ratio of macular FPF to retinal thickness was compared among the three groups with an age-adjusted linear regression model
- Results:
- OHT: both FPF (p<0.05) and FPF/CMT ratio (p<0.01) were significantly elevated
- POAG: FPF was correlated with age and FPF/CMT ratio was significantly elevated (p<0.001)

References
- R McGuigan, “Light Years Ahead”, The Ophthalmologist (2015). Available at: https://bit.ly/2VTM4bL. L Geyman et al., “Non-invasive detection of mitochondrial dysfunction in ocular hypertension and primary open-angle glaucoma”, J Glaucoma, 27, 592 (2018). PMID: 29750714. J Romo et al., “Flavoprotein fluorescence correlation with visual acuity response in patients receiving anti-VEGF injection for diabetic macular edema”, Oxid Med Cell Longev, 2018, 3567306 (2018). PMID: 30159113.
