At a Glance
- Myopia is becoming more prevalent, and it increases the risk of developing sight-threatening conditions, such as cataracts or myopic macular degeneration
- Children are increasingly suffering from myopia, which can have an impact on their education and future prospects
- Four main groups of factors – genetic, environmental, accommodative/vergence and peripheral retinal hyperopic defocus – contribute to development of myopia
- Identifying patients at risk of myopia and acting early can help prevent or delay progression of the condition.
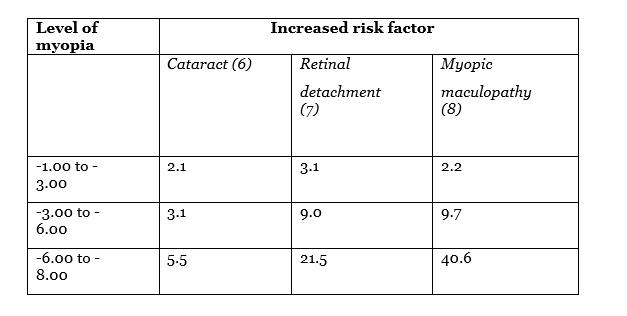
Myopia is more common – and increasing in prevalence more quickly – than ever before. Extrapolating current trends, over half of the world’s population will be myopic by 2050, and one-tenth will have high myopia (1). In some parts of the world, the prevalence is extraordinary: in South Korea, for example, 96.5 percent of 19-year-old males are myopic (2). Furthermore, myopia progression is associated with the development of sight-threatening conditions (Table 1): thus, refractive errors as low as -3.00DS significantly increase the risk of cataracts, retinal detachment and myopic macular degeneration. Indeed, recent studies (3) indicate that over 32 percent of adult Chinese-American myopes have a significant – and untreatable – risk to vision due to myopic macular degeneration. Similarly, myopic macular degeneration is now the leading cause of monocular blindness in Japan (4) and of new cases of blindness in China (5). Hence, myopia soon will be the major factor in sight loss among older people.
Table 1: Higher myopia increases the risk of ocular pathology.
Most concerningly, in many countries the pandemic now extends into younger cohorts; thus, the incidence of myopia in UK children has more than doubled over the last few decades, and now stands at 20 percent. Given that uncorrected childhood myopia can hinder education, it is particularly important to effectively manage this group of patients. Unfortunately, this is not always easy – about 40 percent of myopic children are self-conscious about – or generally dislike – wearing glasses (9).
The implication is clear: managing the pandemic requires treatment not just of older myopes, but also of younger ones who may progress to high myopia and serious sight-threatening disease as they age. What can we do about it? Rational choice of treatment strategies requires that we understand the causes of myopia and that we can identify those children most likely to benefit from treatment.
What makes a myope?
The multifactorial etiology of myopia comprises four main groups of factors: genetic, environmental, accommodative/vergence and peripheral retinal hyperopic defocus (Figure 1). All of these may contribute to an increase in the axial length of the eyeball and hence poor distance vision.
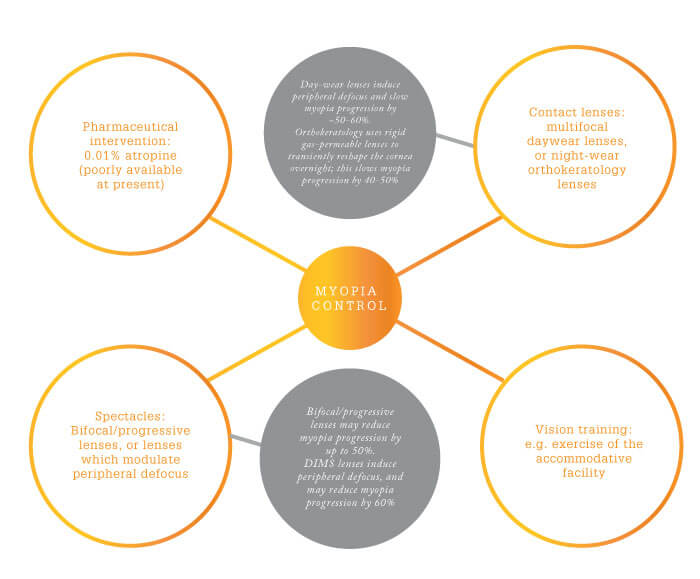
Figure 1: Major factors that contribute to myopia
The genetic contribution to myopia is well-established; where both parents are myopic, the child has a significantly higher risk of myopia (10). At present, we cannot treat the genetic factor – it can only inform our efforts to identify at-risk children. Environmental factors, by contrast, are relatively easy to address; most importantly, the known protective effect of sunlight on the eye and retina (11, 12) suggests a health benefit to outdoor pursuits. Current advice is that children should spend at least two hours outside each day, but this target is rarely reached – in fact, children today spend less time outdoors than ever before. Lack of sleep also is thought to contribute to myopic progression (13), and may be influenced by environmental factors.
Accommodative/vergence factors seem to be important in myopia progression. For example, accommodative lag increases during progression; an increased lag may be evident up to two years before myopia onset (14). However, it is not yet clear whether the lag plays a causative role or only predicts myopia. Other functions of accommodation – such as amplitude, facility and response to blur – also have been implicated as factors or indicators in the progression of myopia (15). For example, we know that accommodation induces an increase in the axial length of the eye (16), the increase being proportional to the amount of accommodation exerted. This suggests that children should avoid holding reading/visual material too close to the eye. Accommodation may also impact myopia via an effect on IOP, as accommodative effort increases pressure (17), which may in turn contribute to myopia progression. Also, the under-accommodation associated with near-vision esophoria may contribute to or predict myopia (18, 19, 20). Thus, bifocal or progressive spectacle lenses have been reported to control myopia progression in esophore children (21). Similarly, some studies suggest that myopia progression may be controlled by spectacles that under-correct myopia (22), although other work indicates the opposite effect – myopia progression and axial elongation (23, 24).
For the purposes of myopia control, however, the most important factor may be peripheral retinal hyperopic defocus. In brief, studies have shown that spectacle-mediated correction of myopia (and uncorrected and undercorrected refractive error) induces hyperopic defocus on the peripheral retina. This defocus stimulates eyeball growth and increases axial length (25), possibly as an attempt at emmetropization. Reduction of hyperopic defocus and induction of myopic defocus is therefore an increasingly important topic in myopia control.
Tomorrow’s high myopes, treated today
To avoid the significant individual and societal impact of myopia progression and related conditions, we should identify at-risk patients in childhood, where possible, and act to prevent or delay progression. Diagnosis of at-risk individuals is informed by genetic factors; by presence of esophoria; and by apparent accommodative/vergence difficulties (26). Age of onset is also significant: early onset myopia (6-7 years of age) is associated with high myopia (>6DS) as an adult. However, the most reliable predictor of myopic onset and progression is a shift away from the normal hyperopic refractive error: future myopes have significantly less hyperopia compared with the average for their age. Furthermore, this diagnostic indicator may be present up to four years prior to the onset of myopia, and a faster shift from the norm is often seen one year before myopia onset (27).
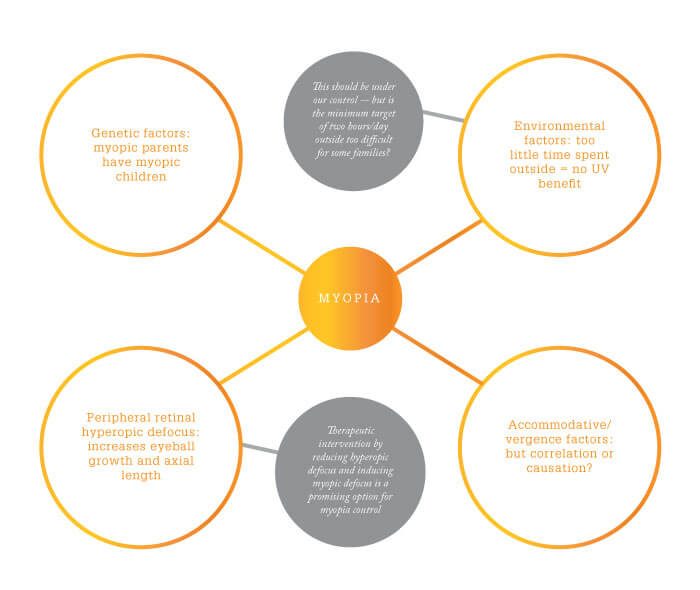
Given the links between myopia and other ocular conditions, parents of all children at risk of myopia or myopia progression should be fully advised regarding available interventions. Today, we have a number of options for myopia control (Figure 2), including pharmaceutical intervention, vision training, and specialized contact or spectacle lenses.
Figure 2: Intervention options for myopia control
Pharmaceutical intervention
The only approved drug relevant to myopic progression is atropine. Its mechanism of action is not fully understood, but it is known to be effective (28): 1 percent atropine slows myopia by 0.68DS/year. That said, atropine has side-effects including blurry vision and reduced accommodation, and there is evidence of rebound myopic progression after treatment cessation (29). In practice, therefore, the application of 1 percent atropine is rather limited. Lower concentrations, however, may be more useful: 0.01 percent atropine has fewer side effects and less rebound myopia; therefore, it may provide longer duration benefit, albeit at the price of slightly lower myopia control (0.53DS/year). Recent studies also indicate that other low doses may be more appropriate (0,05% and 0.025% (30)). Unfortunately, these lower-strength formulations are not widely available and must be compounded specially. There have recently been concerns and mystery about the action of atropine. From the ATOM and LAMP studies (31), despite reducing the rate of progression of myopia, it does not appear to reduce the rate axial length growth. This lengthening of the eyeball is largest risk factor in myopia related pathology. So, the risks of myopia may still be present.
Vision training
The involvement of accommodative/vergence factors in myopia progression suggests that vision training therapy may be an effective intervention. A recent study (32) indicated that accommodation training slows myopia progression compared to controls, at least in younger subjects, but only for a limited period of time. My own experience is that children undertaking vision training for accommodative/vergence difficulties often exhibit reduced myopia progression. Furthermore, this type of training is of broader benefit to these young patients in that it supports reading and studying.
Specialized contact lenses
The orthokeratology approach involves use of reverse-geometry, rigid, gas-permeable (RGP) lenses; when worn overnight, these devices reshape the cornea and provide transient (36 to 48 hour) relief from myopic refractive error. Furthermore, RGPs reduce axial length growth, thereby reducing myopia progression by 40-50 percent compared with no intervention (33). Traditional (day-wear) lenses did not show a similar suppression of eyeball growth, suggesting that the RGP mechanism is likely to be reduced hyperopic defocus on the peripheral retina.
Overnight placement of RGP lenses, however, increases the risk of serious infections, such as microbial keratitis, by 2-6 times as compared with day-wear soft contact lenses (34). This risk is minimal when the device is chosen with appropriate regard to lens design, is carefully fitted, and when the patient is provided with appropriate aftercare. In fact, the rate of contact lens-related events and complications for children is very low (35) – and lower in ages 8-11 than in older children and adults. Nevertheless, parents considering RGPs for their children should be fully informed of all possible outcomes.
Today, however, there is a new contact lens option for inhibiting myopia progression: single-use, disposable soft contact lenses, such as Coopervision’s ‘MiSight’ device (36). These dual-focus, multifocal contact lenses work by presenting the peripheral retina with hyperopic defocus, thus reducing the drive for axial elongation, and reduce myopia by about 50-60 percent compared with controls wearing single-focus contact lenses (37). This outcome is similar to that of orthokeratology; the risk to ocular health is slightly lower when using daily disposable lenses compared with overnight wear. I believe that this soft contact lens advance is one of the most exciting innovations in myopia control.

Specialized spectacle lenses
Spectacle lenses for myopia control fall into two broad categories: (i) bifocal/progressive lenses, and (ii) lenses which alter peripheral defocus. Bifocal/progressive lenses (for example, Myopilux), especially those with a prismatic correction in the near segment, appear to be more effective in children with low accommodation lag and/or esophoria at near. Early reports suggested their effect was not clinically significant, but more recent studies, indicate reductions in myopia progression of up to 50 percent (38).
More exciting, however, is the new generation of spectacle lenses that induce peripheral defocus. The Myovision lens (Zeiss) has had some success, but greater effects are seen with the Defocus Incorporated Multiple Segments (DIMS) spectacle lens. This device, developed by a team at Hong Kong Polytechnic University, is reported to reduce myopia progression by 60 percent (39).
Bomb disposal
The high and increasing incidence of myopia, together with the close link between myopia progression and a number of sight-threatening conditions, points to an explosion of ocular disease in the near future. Defusing this bomb requires that likely pre-myopes are assessed as early as possible, taught good visual habits, and encouraged to decrease the risk of myopic progression by behavioral interventions (for example, by increasing outdoor time). This strategy should slow or prevent myopic progression in at least a proportion of patients.
Once myopia has become established, however, its control requires more significant intervention. First steps include a full assessment of accommodative and vergence factors; patients should be offered vision training where appropriate. Reduction in myopia progression also may be effected by soft contact lenses (such as Misight), or spectacle lenses, such as the new DIMS device. Some patients may benefit from orthokeratology, provided they have access to an experienced practitioner, and with the proviso that parents should be fully informed of the nature of the intervention and appropriate consent obtained. Finally, pharmaceutical intervention may have a role in myopia retardation in the future; for example, if atropine 0.01 percent becomes more widely available, and could be used in parallel with the interventions described above.
Broad and consistent application of the above tactics should enable ophthalmology clinics to minimize the force of the myopia explosion, and shelter patients from the fallout.
What to tell patients – and their parents
- Get outside: at least two hours per day is essential, especially before the onset of myopia
- Get some variety: reduce screen time or near-vision work, and take breaks (remember the 20/20/20 rule – focus on something 20 feet away for 20 seconds every 20 minutes).
- Get further away: don’t hold books or devices close to the eyes.
- Get more sleep: ideally, more than nine hours per day for children.
- Get professional help: children with a high risk of myopia (and especially if they have early onset) should be assessed and offered a myopia control program if appropriate.
References
- The impact of myopia and high myopia. Report of the Joint World Health Organization-Brien Holden Vision Institute Global Scientific Meeting on Myopia, University of New South Wales, Sydney, Australia, 16-18 March, 2015. ISBN 978-92-4-151119- https://www.who.int/blindness/causes/MyopiaReportforWeb.pdf SJ MCullough, L O’Donoghue, and KJ Saunders, “Six-year refractive change among white children and young adults: evidence for significant increase in myopia among white UK children”, PLoS One, 11, e0146332. doi: 10.1371/journal.pone.0146332 (2016). PMID: 26783753. F Choudhury et al., “Prevalence and Characteristics of Myopic Degeneration in an Adult Chinese American Population: The Chinese American Eye Study”, Am J Ophthalmol, 187, 34-42 (2018). PMID: 29288031. Y Suzuki et al., “Risk factors for open-angle glaucoma in a Japanese population”, Ophthalmology, 113, 1613-17 (2006). PMID: 16828504. F Xia et al., “Causes and three-year incidence of irreversible visual impairment in Jing-An District, Shanghai, China from 2010-2015”, BMC Ophthalmol, 17, 216: doi: 10.1186/s12886-017-0603-3 (2017). PMID: 29179756. C Younan et al., “Myopia and incident cataract and cataract surgery: the Blue Mountains eye study”, Invest Ophthalmol Vis Sci. 43, 3625-32 (2002). PMID: 12454028. A Ogawa and M Tanaka, “The relationship between refractive errors and retinal detachment–analysis of 1,166 retinal detachment cases”, Jpn J Ophthalmol, 32, 310-15 (1988). PMID: 3230716. J Vongphanit et al., “Prevalence and progression of myopic retinopathy in an older population”, Ophthalmology,109, 704-11 (2002). PMID: 11927427. Parents’ & children’s views and attitudes about vision correction: Johnson and Johnson Visioncare / Mumsnet (2013). https://tinyurl.com/qh4483o D Kurtz et al., “Role of parental myopia in the progression of myopia and its interaction with treatment in COMET children”, Invest Ophthalmol Vis Sci, 48, 562-70 (2007). PMID:17251451. M Yang et al., “Myopia prevalence in Canadian school children: a pilot study”, Eye, 32, 1042-1047 (2018). PMID: 29391573. H Torii et al., “Violet light transmission is related to myopia progression in adult high myopia”, Sci Rep, 7, 14523: doi: 10.1038/s41598-017-09388-7 (2017). PMID: 29109514. D Jee et al., “Inverse relationship between sleep duration and myopia”, Acta Ophthalmol, 94, e204-210 (2015). PMID: 26031352. D Mutti et al., “Accommodative lag before and after the onset of myopia”, Invest Ophthalmol Vis Sci, 47, 837-46 (2006). PMID: 16505015. D O’Leary and P Allen, “Accommodation functions that predict myopia in young adults”, ARVO Annual Meeting Abstract, May 2004, Invest Ophthalmol Vis Sci, 45, 3515. H Aldossari et al., “Effect of accommodation on peripheral eye lengths of emmetropes and myopes”, Optom Vis Sci, 94, 361-369 (2017). PMID: 28027274. L Yan et al., “Accommodation-induced intraocular pressure changes in progressing myopes and emmetropes”, Eye, 28, 1334-1340 (2014). PMID:25190534. J. Gwiazda et al., “Myopic children with esophoria underaccommodate at near”, 37, S687 (1996). https://tinyurl.com/yx9ll9ne J Gwiazda et al., “Insufficient accommodation and near esophoria: precursors or concomitants of juvenile-onset myopia?” In Tokoro T. (eds) Myopia Updates. Springer, Tokyo (1998). ISBN: 978-4-431-66961-6. K Chung and E Chong, “Near esophoria is associated with high myopia”, Clin Exp Optom, 83, 71-75 (2000). PMID: 12472457. G Fulk et al., “A randomized clinical trial of bifocal glasses for myopic children with esophoria: Results after 54 months”, Optometry, 73, 470-6 (2002). PMID:12365670. Y Sun et al. “Effect of uncorrection versus full correction on myopia progression in 12-year-old children”, Graefes Arch Clin Exp Ophthalmol, 255, 189-195 (2017). PMID: 27796670. K Chung et al., “Undercorrection of myopia enhances rather than inhibits myopia progression”, Vision Res, 42, 2555-59 (2002). PMID: 12445849. D Adler and M Millodot, “The possible effect of undercorrection on myopic progression in children”, Clin Exp Optom, 89, 315-21 (2006). PMID: 16907670. K Allinjawi et al., “Peripheral refraction with different designs of progressive soft contact lenses in myopes”, F1000Res, 5, 2742 doi: 10.12688/f1000research.9971.1 (2016). PMID: 28163898. L O’Donoghue et al., “Risk factors for childhood myopia: findings from the NICER Study”, Invest Ophthalmol Vis Sci, 56, 1524-30 (2015). PMID: 25655799. K Zadnik et al., “Prediction of juvenile-onset myopia”, JAMA Ophthalmol, 133, 683-9 (2015). PMID: 25837970. P Turnbull and S Khanal, “Atropine – efficacy & possible mechanisms for myopia control”, (2017). https://tinyurl.com/y4g9lfxw A Chia et al., “Five-Year Clinical Trial on Atropine for the Treatment of Myopia 2: Myopia Control with Atropine 0.01% Eyedrops”, Ophthalmol, 123;391-399 (2016). PMID: 26271839. JC Yam et al., “Low-Concentration Atropine for Myopia Progression (LAMP) Study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control”, Ophthalmol, 126, 113-124 (2019). PMID: 30514630. M Bullimore and D Berntsen, “Low-dose atropine for myopia control: considering all the data”, JAMA Ophthalmol, 136, 303 (2018). PMID: 29423500. H Price et al., “The Cambridge anti-myopia study: variables associated with myopia progression”, Optom Vis Sci, 90, 1274-83 (2013). PMID: 24100478. M Smith and J Walline, “Controlling myopia progression in children and adolescents”, Adolesc Health Med Ther, 6, 133-40 (2015). PMID: 26316834. N Cheung et al., “Emerging trends in contact lens-related infections”, Curr Opin Ophthalmol, 27, 327-32 (2016). PMID: 27176217. M Bullimore, “The safety of soft contact lenses in children”, Optom Vis Sci, 94, 638-646 (2017). PMID: 28514244. “Four-year study: Pioneering contact lens approach slows myopia progression in children”, (2018). https://tinyurl.com/y4mvqr2h Lam et al., “Defocus incorporated soft contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: A 2-year randomised clinical trial”, B J Ophthalmol, 98, 40-45 (2013). PMID: 24169657 D Cheng et al., “Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: three-year results of a randomized clinical trial”, JAMA Ophthalmol, 132, 258-64 (2014). PMID: 24435660. ‘Defocus Incorporated Multiple Segments (DIMS) spectacle lens for myopia control awarded the chapionship in Geneva’s Invention Expo’: Hong Kong Polytechnic University Media Release (2018). https://tinyurl.com/y2mkzlug
