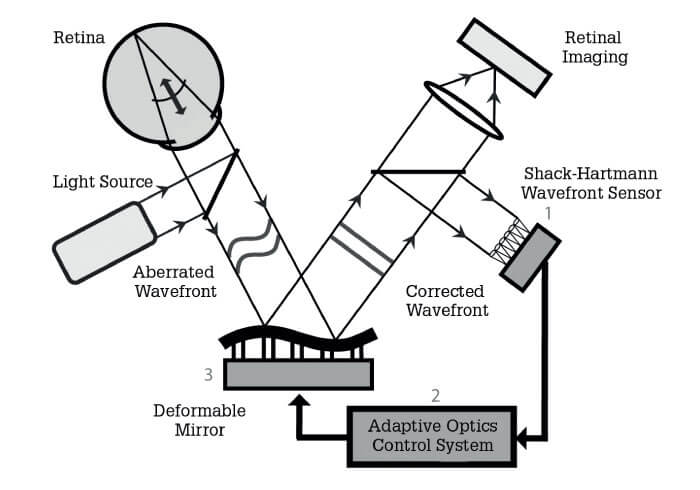
- Adaptive optics compensates for the intrinsic optical errors of ocular tissues, thereby providing cellular-level resolution of the retina
- Advanced adaptive optics systems now permit dynamic monitoring of retinal cell function in real time
- As this technology moves from the R&D environment to the clinic, the benefits to patients are becoming increasingly evident; near-term applications include improved assessment of acute retinal neuritis, commotio retinae and retinal gene therapy trials
- Longer-term aims include the development of a visual function test that will permit detailed monitoring of both photoreceptors and retinal vasculature, thus guiding management of both ocular and systemic vascular disease.
I can trace my interest in vision science to the moment when, as a post-doc, I found out I was color-blind. How could I have lived for 26 years without realizing this? More specifically, how had my visual system compensated for it? These types of questions kicked off my career, and led to my current fascination with the assessment of visual and retinal function. It’s a field with significant unmet needs; after all, standard tests are not only subjective but also require patients to concentrate on reporting visual stimuli for unreasonably long periods (up to an hour, once you include repeat tests). A more objective approach is available, but this involves placing an electrode on the eye and using very bright lights as stimuli, which is uncomfortable for the patient. These tests are still reliant on the patient remaining still, fixating and not moving.
Whatever approach you choose, the fact remains that standard technologies cannot detect pathological change before irreversible retinal damage. The most sensitive imaging test in clinics now, optical coherence tomography (OCT) is limited to identifying change at the level of cell clusters rather than of individual cells. Therefore, moving towards patient centric treatments, we need better tools to assess and monitor the retina – ideally, systems that can both provide stimuli tuned to the specific response ranges of given cells, and also objectively measure the subsequent responses of those individual cells. Furthermore, cellular level resolution would help us more precisely understand disease processes – and enable earlier interruption of those processes. We ask the question, is it possible to develop a clinically relevant technology with these attributes?
The main challenge in developing a system that could image the retina at single-cell resolution is the optical quality of the human eye. Many of us wear glasses to improve our sight, but we also need to remember our eyes are filled with liquid. Looking through a bottle of liquid, even if it is clear water, the view is still distorted. If you remember the warm summer nights, the streetlights often seem to have halos or little spears of light coming from them? Those effects are caused by water molecules in the air refracting the light. The same thing happens in the eye, which of course you can think of as a couple of sacs of liquid fronted by more liquid in the tear film. When you are looking out at the world, your brain naturally compensates for these artefacts – but when we look into the eye to examine the retina, we have no equivalent compensation mechanism, and so our image of the posterior chamber is impaired. In fact, this problem was very similar to that faced by astronomers seeking to visualize remote stars – and the answer they found became the basis of an extraordinarily promising approach to retinal imaging and vision assessment.
In brief, adaptive optics is a “bolt-on” technology, compatible with any retinal imaging system; its most significant attribute is that it can measure the pathways of light entering and leaving the eye, establish the optical errors that arise and compensate for them. There are various approaches to this broad end; a common method is to use a deformable mirror – in which as many as 200 elements are employed to change the mirror topology – to adjust for ocular optical error (see Figure 1). But whatever the exact approach, the end result is clear – resolution at the fundus of about 2 microns. Compare that with the 15-micron resolution limit associated with the best alternative imaging technologies! And adaptive optics systems continue to improve; today, with better computers and better cameras, we can take videos at incredibly high-frame rates while continually compensating for the optical errors of the eye. And that allows us to monitor not just the structure, but also the function of the living retina over time, opening up a range of exciting possibilities.
With adaptive optics, we can now collect unparalleled images of outer retinal cells, photoreceptors and RPE; we can also gain exquisite views of the blood vessels in the inner retina. In particular, we can see and track individual cells, and precisely quantify cell loss in a given region over time. For example, if you noted there were 100 cells in one area 12 months ago, but today there are 90, you can start thinking about clinical decisions much earlier than would otherwise be possible. It’s remarkable to think that just a few years ago this information would only be available from explanted eyes. The ability to track functional changes over time will be key in addressing one of the biggest challenges in retinal imaging, namely the presence of non-perfused blood vessels.
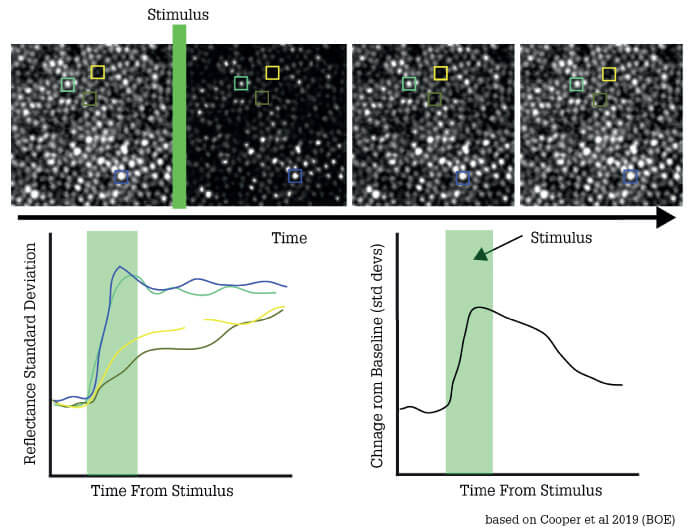
At present, the best non-invasive method of mapping retinal blood vessels is optical coherence tomography angiography (OCT-A) – but this can’t capture non-perfused vessels, which do not show up on the OCT-A image. Furthermore, OCT-A cannot establish if the apparent disappearance of a vessel from one time point to the next is due to genuine pathology, or a reflection of normal physiological regulation of capillary blood flow. By contrast, adaptive optics can monitor flow in real time and establish if vessels are genuinely blocked or merely controlling the blood flow according to physiological requirements (see Figure 2).
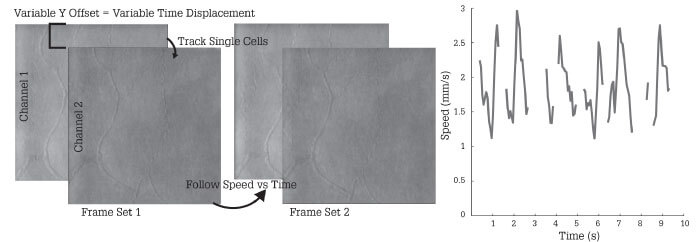
This capability is tremendously important for my own research, which focuses on the vascular system; adaptive optics allows me to study even the smallest blood vessels of the eye and monitor their function dynamically. My great hope is to develop a highly objective, repeatable visual function test that will permit detailed monitoring of both photoreceptors and retinal vasculature. The aim is to make the test so easy and robust that you can have the patient sit down, and chat to them normally while acquiring the necessary data. The idea is to relieve them of the need for unbroken concentration on small flashing lights. Thus, removing subjectivity, and keeping the patient’s experience as comfortable and positive as possible. Furthermore, we believe that adaptive optics equipped instrumentation can also reveal information about how the vascular system as a whole might be changing. Correcting the eye’s aberrations also allows visualization and testing of the eye’s vascular function. Such information could assist in the diagnosis and management of non-ocular diseases, such as dementia. In this way, adaptive optics technology could inform patient care beyond ophthalmology.
It’s true that adaptive optics is still primarily a research tool; nevertheless, we are on the cusp of generating solid evidence for how adaptive optics may directly affect clinical outcomes, not least in the gene therapy field. The first attempts at retinal gene therapy back in 2008 gave highly variable clinical improvements, with some patients responding positively and others negatively. One the biggest challenges was not fully understanding he cellular substrate in each individual patient, and what cells were even present that could be saved. The last several years has seen an explosion in number of gene augmentation trials, many of which are using adaptive optics technology in the early stage to try and understand the underlying cellular structures in these diseases and what improvements treatment may be bringing. This allows the trials to assess whether the treatment truly has zero effect, or a small effect that does not meet the therapeutic threshold present in standard functional tests. And that can help you decide whether to scrap the investigational drug altogether and start again, or just tweak the system (for example, to improve expression efficiency). Such insight is valuable, because in many cases starting a new drug development program from scratch is far more expensive than modifying an existing one.
Overall, I believe that as we learn more about the technology and the eye, clinical benefits will inevitably emerge. My own research suggests that near-term applications are likely to be seen in fields including commotio retinae and retinal neuritis. The former condition, which is associated with head injuries such as those seen in boxing, arises when the retinal photoreceptors are damaged in a discrete area. The damage is sometimes clearly visible by normal examination – but sometimes it isn’t.
When we tested a cohort of patients who had been complaining about blind spots, but in whom doctors could find no retinal damage with normal fundus exams and microperimetry, our adaptive optics system revealed damage that precisely corresponded to the blind spots described by the patients. The ability of this technology to reveal lesions well below the resolution capability of other approaches is likely to become a great diagnostic asset. Similarly, in retinal neuritis – a collection of conditions generally linked to an autoimmune disease of the eye which can result in temporary or permanent sudden-onset blind spots. The imaging afforded by adaptive optics permits us to establish how much damage has been done and provide better data to speculate about whether the lesions are likely to recover or if vision lose is irreversible. This is important in helping to counsel and prepare the patient accordingly, rather than waiting to see if vision will return when it cannot.
Part of the interest in the field can be explained by a 2015 US NIH initiative with the bold objective of finding cures for vision disorders within 15 years. The first phase of this initiative focused on developing better tests to image the retina and objectively test its function. In support of this, the NIH disbursed multi-million-dollar grants to five different research collectives, each of which proposed to collaborate in the field of adaptive optics. The deal is that each group should work completely openly: they must make their data public each year, collaborate with the other three groups, and support other researchers in the field who were not lucky enough to receive the NIH funds.
Unfortunately, parallel development of different technologies (1) can raise difficulties with regard to standardization: the software developed for one system, for example, won’t be exactly right for a different system. But I don’t believe this will halt the development and ultimate clinical application of adaptive optics; indeed, those of us who work in the field are actively meeting this challenge through collaboration and co-operation. My experience is that colleagues are happy to share their analysis software as best they can and advise on how to make it work with other systems. Perhaps there are pragmatic reasons for this – the more similar we make our respective systems, the easier it will be for us all to participate in large clinical trials (because if the instruments at different sites are reasonably interchangeable, all those sites can participate in the same trial). Nevertheless, achieving standardization across the adaptive optics field remains a significant challenge without a significant industry partnerships.
We also need to address the fact that we simply don’t yet know enough about the technology’s potential to fully understand how best to use it in a clinical context – indeed, every time we put a patient in front of an adaptive optics machine, we see new things. For example, we used to think that little bumps on the inner retina were indicative of pathology – but now we find that these features occur with equal frequency in young, healthy people.
We just haven’t looked at enough eyes to know what is normal and what is not. But, in a way, that’s also exciting; we are in great position to move forward, because at this point we are limited only by our ideas, not by technology or by computer processing power. We can acquire and process larger and larger amounts of information more and more quickly, and this is providing better and better diagnostic and research capabilities. Alongside this comes the need to find a balance between examining the entire retina of a patient or concentrating on particular cells; and between the time a patient can sit in front an adaptive optics instrument and the amount of imaging information we are asking for. Ultimately, we’ll probably be guided by pragmatic considerations, such as the kinds of conditions we can actually diagnose, and the extent to which the information we can get will change disease management, patient care and clinical outcomes.
I am confident that adaptive optics systems, although not yet approved by US or EU regulators, will soon move from the research environment into the clinical setting. Commercial prototypes are already being developed by several companies; I am certain that we will see one or more devices reach the market in years to come – with profound benefits for eye care.
The field of adaptive optics is characterized by diverse approaches to diverse applications. Parallel evolution of technology has resulted in non-standardized systems being developed to address a variety of different research questions. The main applications and technical approaches have been outlined in detail elsewhere (1); some are summarized below.
Adaptive optics applications
- Vision correction:
- Simulate effect of proposed IOL design before manufacture, to save time and cost during development
- Simulate impact of vision correction pre-surgery, to ensure optimal outcome
- Support development of new device designs; for example, multifocal contact lenses to slow myopia progression in children
- Identify patients who are good candidates for particular surgical approaches, such as monovision presbyopia correction
- Retinal imaging:
- Single cell resolution permits diagnosis and monitoring at early stages of disease, and assists development of normative databases
- Allows measurement of retinal blood flow changes in response to visual stimulation
- Permits comparison of cones in different regions and times, and of foveal development in healthy and diseased retina
- Visualizes photoreceptors out to 30 degrees in temporal and nasal retina
- Supports more detailed interpretation of OCT images and more quantitative analysis of retina over time
Adaptive optics technologies
- Deformable mirror (various models)
- Advantages: Large stroke helps correct large aberrations; wavelength-independent; more affordable; can correct the broad variety of wave-front errors found in living eyes; permits closed-loop operation (in which the deformable mirror is continuously updated during the evaluation)
- Disadvantages: Limited to representing smooth surfaces
- Liquid crystal spatial light modulator (LC-SLM)
- Advantages: Flexibility and ease of use; high resolution (allows reproduction of complex wave fronts, simulation of diffractive and multifocal optical elements);
- Disadvantages: Works best with narrow bands of light; subject to chromatic artefacts; polarization.
References
- S Marcos et al., “Vision science and adaptive optics, the state of the field”, Vision Research, 132, 3 (2017). PMID: 28212982.
- K Stepien et al., “Subclinical photoreceptor disruption in response to severe head trauma”, Arch Ophthalmol, 130, 400 (2012). PMID: 22411676.
