The great diagnostic potential of SPECTRALIS® OCT angiography*
The unveiling of SPECTRALIS®OCT angiography* is one of the highlights for Heidelberg Engineering in 2016. Its Managing Director Dr. Kester Nahen explains the distinctive features and new possibilities of this technology.
Dr. Nahen, what is OCT angiography and how does it work?
OCT angiography (OCT-A) is an application of optical coherence tomography (OCT), which, as we
all know, documents differences in reflectivity within tissues such as the
retina. In contrast to traditional OCT, OCT-A analyzes not only the intensity
of the reflected signal but also the time changes in the reflection caused by
moving particles – for example erythrocytes flowing through vessels. These
changes in the OCT signal, measured by repeatedly capturing OCT image (B-scans)
at each point on the retina, allow the creation of an image contrast between
the perfused vessels and the surrounding tissues, which does not display any
time changes in the OCT signal due to the lack of movement.
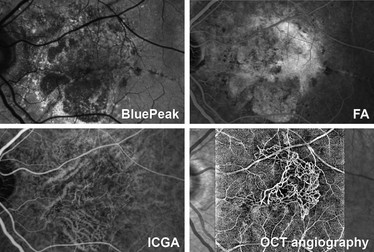
How does OCT angiography differ from fluorescence angiograph (i.e. fluorescein and
indocyanine green angiography)? Is there a decisive advantage?
Compared with traditional fluorescence angiography, this new method offers both clinical and
practical advantages, although it does come with its physical limitations. One
very significant advantage of OCT-A is that it does not require a contrast
agent, so the associated risks of dye injection are eliminated. For this
reason, OCT-A can be performed more frequently than fluorescence angiography.
In addition, OCT-A makes it possible to visualize distinct vascular networks at
different depths in the retina. Fluorescence angiography does not offer this
kind of spatial resolution. Speaking figuratively, OCT -A allows clinicians to
move through the vascular network of the retina layer by layer.
What existing technology is employed and what has been additionally developed or utilized?
OCT angiography is based on the established SPECTRALIS imaging platform. To achieve the scanning
speed needed for OCT-A, we have introduced a new OCT module called OCT2, which
offers improved image quality across the whole depth of field at a considerably
higher capture speed of 85,000 Hz. OCT2 is thus well suited for advanced
applications such as OCT-A.
What is the future potential of OCT angiography?
OCT-A offers great diagnostic potential for ophthalmology, particularly in terms of identifying
and classifying degenerative changes in the perfusion behavior of the retinal
vasculature and following these changes over time. Many experts agree that the
procedure offers new, treatment-relevant information in a wide range of
applications and in some cases could even replace fluorescence angiography.
Consider, for example, the numerous follow-up examinations involved in the
course of intravitreal anti-VEGF therapy. I see a huge potential for OCT-A to
track how degenerated vessels respond to treatment. However, at present, more
intensive clinical investigations are required before specific recommendations
can be made.
...and where are its limits?
Despite the enthusiasm, it is important to recognize that the technology does have some
limitations. Static or very slow flow phenomena such as capillary leakage and
polyps are not well visualized on OCT-A. In such cases, it is not possible to
generate sufficient motion contrast. With OCT-A, it is also not possible to
differentiate between arteries and veins in the same way as with the inflow of
dye in fluorescence angiography. In addition, interpreting the 3D images may
pose a challenge as this is still a relatively new technique.
Will OCT angiography replace fluorescence angiography completely?
From our perspective, OCT-A and fluorescence angiography offer diagnostically complementary
information. Both procedures are of diagnostic significance and this will not
change in the future. It remains to be seen how great the overlap is. In some
applications, however, OCT-A does have the potential to replace fluorescence
angiography. This is a very exciting development for us and we are supporting
the work of various research groups to establish practice guidelines.
What are the clinical applications of OCT angiography? Does it have implications for pharmaceutical research?
The development of OCT-A is a clear indication of the rising demand for diagnostic innovation.
Early users confirm that the combination of OCT-A with other diagnostic imaging
modalities will offer an improved understanding of many clinical conditions. For
years, the use of multimodal imaging has allowed a more targeted approach for
individualized treatment. The high-resolution, 3D visualization of the
vasculature provided by OCT-A is extremely interesting for pharmaceutical
research, because it can help map functional changes resulting from new
therapies. The noninvasive nature of this imaging modality is also attractive
for use in clinical studies. We are in contact with pharmaceutical companies regarding
OCT-A as well as other proprietary imaging modalities.
What does it offer the physician and the patient? Who benefits the most?
The identification of degenerative changes in the microvasculature, which is not possible with other
procedures, would be a huge advantage for the physician. From a patient’s perspective,
the possibility of an earlier, more comprehensive diagnosis and the opportunity
to avoid many fluorescent dye injections are both significant advantages.
Is it just for hospital use or could it also be utilized by physicians in private practice?
At this time, OCT-A is still in a clinical evaluation phase. To achieve reliable outcomes that can
later be transferred to the use of OCT-A by private practitioners, experience
needs to be gained under trial conditions. For this reason, OCT-A is currently
mostly used in clinical settings. As previously mentioned, OCT-A offers great
diagnostic potential for ophthalmology, particularly in terms of identifying
degenerative changes in the perfusion behavior of the retinal vasculature. As
such, OCT-A is set to become an important tool for physicians in private
practice in the future, just as OCT already is today. It is a subject that the
ophthalmology world should be paying close attention to.
Where did the idea for OCT-A arise? Was it in your own R&D department?
Good products require input from company research and development as well as from external
collaborators. To bring an innovative product to market, we work closely with different
groups, both at a pure research level and in applied clinical research. We have
been toying with the idea of imaging vascular perfusion for many years now.
Take, for example, the Heidelberg Retina Flowmeter (HRF), launched in the early
1990s, or our extensive experience in the field of perfusion measurement and
angiography with the SPECTRALIS HRA. Thus, I would describe OCT-A as a result
of continuous innovation in our product range.
How long does it take for an idea like this to go from concept to a clinically useful product?
That’s an interesting question, and one that has no simple answer. At a minimum, several years are
required. We constantly consider the limitations of existing technologies and look
for opportunities to improve diagnostic performance and clinical flow. We have
identified the limitations of conventional angiography and have been working on
developing a number of advancements like OCT-A for some years. The new OCT2
module provides us with the hardware necessary to transform these ideas into
marketable products.
What were the greatest challenges so far in developing OCT-A?
Eye movements pose one of the greatest technical challenges in ophthalmic OCT imaging. In OCT-A,
reflection must be measured repeatedly at exactly the same point on the retina.
In addition, a large number of densely spaced cross-sections need to be
acquired to achieve a high-resolution 3D dataset. Even with the fastest
commercially available OCT components, OCT-A datasets can suffer from motion
artifacts unless effective eye tracking is used. This poses a real problem and
risk from a diagnostic perspective. To avoid this, we use a technology called
TruTrack Active Eye Tracking, which detects eye movements during acquisition
and only saves datasets that were definitely captured without any eye movements.
It is a kind of built-in quality assurance system. Moreover, the TruTrack
Active Eye Tracking system repositions the OCT scan at the correct location on
the retina if an eye movement has occurred. This patented technology ensures
that the dense 3D volume scans required for OCT-A can be taken without motion
artifacts that would otherwise diminish the clinical value of OCT-A.
What remains to be done? Can the module be refined even further?
Of course! If we just think back to traditional OCT, we have been refining and expanding the areas of
application and functions for years, and the same will undoubtedly be true for
OCT-A. Although the technology is very exciting and we are enthusiastic about
its possibilities, it is still in its infancy.
Do you think the technology could also find use outside ophthalmology?
OCT is already employed in other fields such as neurology, dermatology, and cardiology. As
these specialties also often deal with the issues surrounding vascular
perfusion, OCT-A is of great interest to doctors in those fields as well.
Does your company have a specific development philosophy, and how would you describe it? What can we expect next?
We are celebrating our 25th anniversary this year. Looking back over the years, two aspects become
particularly clear. For one, we only develop and market products if we are
completely convinced that they are clinically relevant and will really help us
to advance diagnostic imaging. We are constantly breaking new ground when it
comes to new technologies. However, we always follow the principle of
validating new procedures and forming a deep understanding of them before we
commercialize new technologies. This process usually takes years, but it
ensures that our technologies are not sold before they are truly ready. Our
customers know that if Heidelberg Engineering markets something, it has been
thoroughly tried and tested. It is also important to us to be able to offer our
customers long-term solutions. The SPECTRALIS OCT is a shining example. We do
not see it as a product with a short lifecycle, but as a flexible platform that
can be expanded and updated with state-of-the-art technology. The new OCT2
module, which serves as the basic platform for OCT-A, not only is available for
new devices, but also can be used to upgrade a range of existing devices. In
our opinion, this sustainability is an outstanding source of added value to our
customers.
This interview was conducted by Susanne Wolters and as originally published in German in the journal CONCEPT Ophthalmologie, 06/2015.
*The OCT Angiography Module is under development and not for sale yet.

Heidelberg Engineering













