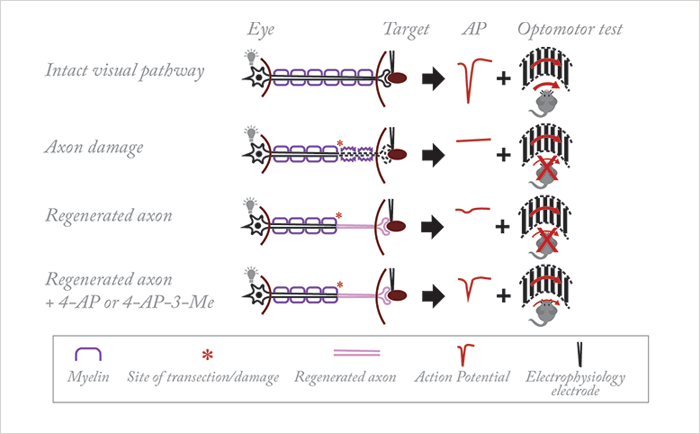
Myelin is a wonderful mixture of proteins and lipids – the mere fact that oligodendrocytes (in the central nervous system) and Schwann cells (in the peripheral nervous system) wrap axons in myelin means that neural signaling can propagate speedily over far greater distances than is possible in their unmyelinated counterparts. What’s interesting is that today, in mice, it’s possible (with the right cocktail of neurotrophic factors) to regrow damaged optic nerve fibers – but to get them to function requires myelin – or, as it turns out, the presence of a voltage-gated K+ channel blocker like 4-aminopyridine (4-AP). Fengfeng Bei, Henry Hing Cheong Lee and colleagues (1) are able regrow optic nerve fibers in mice with transected optic nerve tracts – either by deleting the PTEN and SOCS3 genes, or by overexpressing osteopontin (OPN), insulin-like growth factor-1 (IGF-1) and ciliary neurotrophic factor (CNTF) genes (Figure 1). Either approach achieves the same feat: retinal nerves that grow toward and terminate in the superior colliculus of the brain, forming functional synapses when they get there. The problem is, they don’t restore any semblance of visual acuity. Why? No signal reaches there, because of a lack of myelination.
What made them reach that conclusion? The mice with the regrown retinal nerve fibers weren’t able to express any visual behavior, as assessed by optomotor function tests (the rotation of a grating in front of mice should elicit head movement – so long as they can see it in the first place). Mice with transected optic nerve tracts showed poor optomotor responses (unsurprisingly) – but so did the mice with regenerated axon tracts. Their next step was to perform immunohistochemistry work – which confirmed that these axons lacked a myelin sheath. Multiple sclerosis is a disease that’s characterized by demyelination, and an EMA-approved drug exists that has been shown to significantly improve conduction in demyelinated axons in patients with the disease: 4-AP. Bei et al. wondered if the drug could improve conduction of the demyelinated axons present in their mouse regeneration model. 4-AP administration in these mice resulted in significant improvements in their optomotor function tests, and electrophysiological experiments confirmed this (Figure 1).
But 4-AP has a relatively short half-life – 6 hours in humans – and is associated with an increased incidence of unpleasant side effects, such as seizure and hypersensitivity reactions. One alternative might be 4-aminopyridine-3-methanol (4-AP-3-Me), which appears to be more potent than 4-AP at increasing axonal conduction, and may have a more favorable side-effect profile – the study authors found that 4-AP-3-Me, even at a lower dosage, improved optomotor performance and increased electrophysiological conduction, than even 4-AP achieved. Of course, another approach could be to promote remyelination by blocking activity of the protein LINGO-1, a negative regulator of myelination. This method means that you no longer depend on drug bioavailability for axon conduction, and this approach has already shown promise for the treatment of patients with optic neuritis, a condition often associated with multiple sclerosis. Taken together, Bei et al’s work confirms that, following optic nerve damage (as can occur in humans following trauma, or part of the natural history of glaucoma) you not only have to regenerate the axons, but you also have to restore conduction, if you want to restore visual input into the visual systems of patients.
References
- F Bei et al., “Restoration of visual function by enhancing conduction in regenerated axons”, Cell, 164, 219–932 (2016). PMID: 26771493.
