- SAP is considered to be the gold standard technique for identifying visual field loss in patients with glaucoma
- However, there is debate over whether changes seen on OCT alone can be used to support treatment decisions in glaucoma
- I believe that OCT can – and should – be used, because it provides a window of opportunity into early management of the disease
- Presenting supporting evidence, I justify my position and explain how basing clinical decisions on OCT (even in the absence of a concomitant visual field defect) can improve outcomes for patients
The introduction of imaging with optical coherence tomography (OCT) has allowed clinicians to acquire quantitative information about the eye structures affected by glaucoma with unprecedented detail. Despite this fact, clinicians are often confused about how to use OCT information in clinical practice. Should we make treatment decisions based only on OCT? I am asked this question very often and so below I provide some evidence of why I believe OCT should be used in clinical decision-making – even in the absence of concomitant visual field loss.
I will use three main points to justify my position:
- A diagnostic test should only be used if its results have a reasonable chance of impacting decisions about treatment.
- Contrary to some prevailing thoughts, the value of OCT for monitoring glaucoma over time is predominantly based on the fact that the test actually disagrees substantially with standard automated perimetry (SAP).
- OCT provides a window of opportunity into the management of glaucoma.
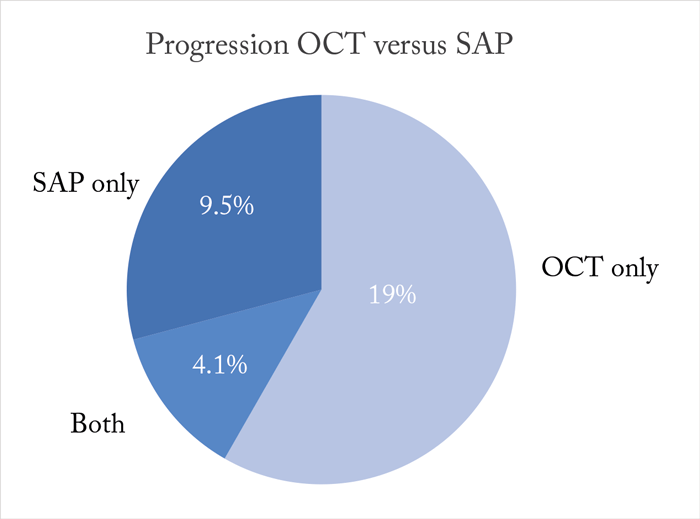
However, I should note that the above does not necessarily imply that changes seen on OCT will always lead to modifications in treatment. Clearly, all treatment decisions need to be based on many factors, including the severity of disease and risk of functional impairment, as well as the patient’s age, life expectancy, and risks and potential side effects of therapy. Changes seen on OCT may also lead to modifications in management, for example, by indicating a need for more frequent follow-up visits. However, the main point to drive home is that unless a clinician is willing to make changes in management based on progressive changes that are seen only on OCT and in the absence of concomitant loss of visual function, OCT will hardly have any value as an ancillary test. My second point should also be quite obvious. If OCT results agreed with visual field assessment in most patients, there would be little justification to acquire another test besides the traditional gold standard, which is SAP – in other words, one would suffice. However, the evidence is very clear that agreements between OCT and SAP in detecting change over time are the exception rather than the rule. Recently, my colleagues and I followed 462 glaucomatous eyes from 305 patients, with spectral-domain OCT and SAP performed at approximately every 3–6 months over a mean period of 3.6 years, to investigate the relative odds of detecting progression by one test versus the other (1). We found that OCT and SAP agreed on progression in only 4.1 percent of the eyes, whereas 19.0 percent of glaucomatous eyes showed progression only on OCT and 9.5 percent showed progression only on SAP (Figure 1).
The need to act – based on progression seen concomitantly by OCT and SAP or by SAP alone – is generally not under debate, as long as the changes are considered repeatable. However, almost 20 percent of patients with glaucoma exhibited changes in OCT that were faster than age-related change (95th percentile established from healthy eyes) and were seen in the absence of concomitant SAP changes. Once again, if we are not willing to make decisions based on OCT only, are we to ignore progression seen in this large proportion of patients? We – and others – have previously published on the reasons for the frequent disagreements between OCT and SAP. Such disagreements become easy to understand once one considers the properties of the tests, such as different scaling and issues related to variability. Importantly, the changes seen on OCT, such as slopes of retinal nerve fiber layer thickness loss over time, have been shown to predict future development of visual field loss (2)(3) and be associated with a decline in patient-reported quality of life in glaucoma (4). At this point, it is worth asking: “Can’t I just wait until I see a change in the visual field to start or modify treatment?” In other words, do I need to bother with early treatment? Such questions bring us to the third point, which is the window of opportunity that OCT provides.
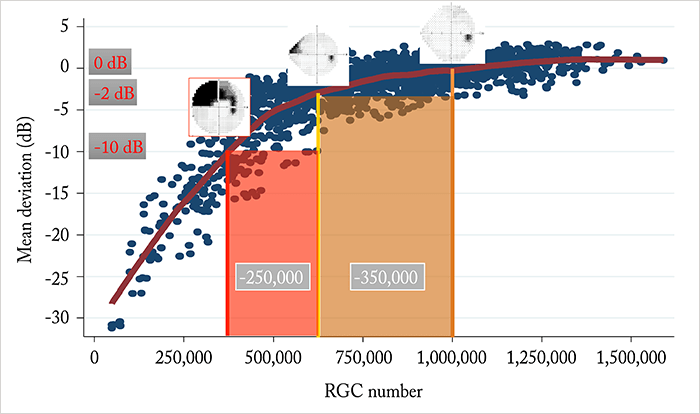
Because of the nonlinear relationship between visual field loss measured by SAP and retinal ganglion cell (RGC) number, it can take a substantial loss of RGCs for an initial visual field defect to become clearly apparent. OCT can frequently detect progression before that. However, once such a defect is apparent on SAP, it actually takes a smaller amount of RGC loss for the defect to progress to significant functional impairment (Figure 2). In other words, once a visual field defect is present, less change can be tolerated, meaning that more aggressive treatment will likely be needed to slow down the disease to avoid progression to functional impairment. On the other hand, if treatment is initiated earlier – within the window of opportunity offered by OCT – there will be a greater tolerance in the allowed rates of change that can prevent future functional impairment, meaning that treatment can potentially be less aggressive.
In conclusion, we know as clinicians that treatment decisions should never be based on a single test; candidly, that’s common sense and is not the point of this discussion. However, there is evidence to suggest that changes seen only on OCT in the absence of functional loss have important prognostic significance for patients with glaucoma or those suspected of disease. Ignoring these changes based on claims of lack of agreement between OCT and SAP reflects a misunderstanding of the structure-function relationship in the disease. If the structural changes seen on OCT are real, repeatable and faster than age-related loss, we should consider them in making treatment decisions. If we fail to do so, we could be missing a window of opportunity to improve outcomes for our patients.
Felipe Medeiros is Professor of Ophthalmology and the Ben and Wanda Hildyard Chair for Diseases of the Eye, University of California, USA. Financial Disclosure: Research support from Carl-Zeiss Meditec, Heidelberg Engineering, Topcon, Reichert, Sensimed, Allergan and Alcon; Consultant to Carl-Zeiss Meditec, Heidelberg Engineering, Reichert, Allergan and Alcon.
References
- RY Abe et al., “The relative odds of progressing by structural and functional tests in glaucoma”, Invest Ophthalmol Vis Sci, 57, OCT421–428 (2016). PMID: 27409501. A Miki et al., “Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes”, Ophthalmol, 121, 1350–1358 (2014). PMID: 24629619. M Yu et al., “Risk of visual field progression in glaucoma patients with progressive retinal nerve fiber layer thinning: a 5-year prospective study”, Ophthalmol, 123, 1201–1210 (2016). PMID: 27001534. CP Gracitelli et al., “Association between progressive retinal nerve fiber layer loss and longitudinal change in quality of life in glaucoma”, JAMA Ophthalmol, 133, 384–390 (2015). PMID: 25569808. FA Medeiros et al., “A combined index of structure and function for staging glaucomatous damage”, Arch Ophthalmol, 130, 1107–1116 (2012). PMID: 23130365. RS Harwerth et al., “Linking structure and function in glaucoma”, Prog Retin Eye Res, 29, 249–271 (2010). PMID: 20226873.
