Eyedrops don’t have to be preserved. Given the right formulation and container, topical glaucoma medications can be made without the preservatives. It’s clear that a significant proportion of patients that receive chronic therapy with preservative-containing (PC) eyedrops experience ocular side effects (1). For example, many patients with glaucoma or ocular hypertension have ocular surface disease (OSD), and that’s driven by the preservatives present in the eyedrops – principally the quaternary ammonium surfactant, benzalkonium chloride (BAC) (2). An increasing number of glaucoma medications have been associated with an increased frequency of severe dry eye symptoms and decreased emotional quality of life (3). BAC’s damaging effects on the ocular surface are many (reviewed in the previous issue), and the OSD it can produce can be irritating, painful and significantly impact upon patients’ quality of life. Furthermore, both patient dissatisfaction and the occurrence of adverse events have been shown to correlate with disease progression (4). However, in the past, it was thought that BAC was required in topical glaucoma medicine formulations as a penetration (and therefore efficacy) enhancer (5). So what is the current evidence for and against BAC? A number of clinical studies have compared the efficacy of preserved and preservative-free (PF) topical glaucoma therapies. Hamacher et al. (6) examined the efficacy of PF- and BAC-preserved tafluprost in a 4-week crossover study in patients (n=43) with open-angle glaucoma (OAG) or ocular hypertension, and found that these formulations had equal efficacy (p=0.96). Shedden et al. (7) also found no difference in the IOP-lowering effect of PF- and BAC-preserved dorzolamide 2%/timolol 0.5% fixed combination eyedrops, in a trial that randomized 261 glaucoma patients to either drug. Clearly, the presence of preservatives has no effect on the efficacy of these anti-glaucoma drugs. PF formulations can be of benefit to patients that experience ocular surface issues associated with PC prostaglandin formulations. Uusitalo et al. (8) investigated the tolerability and IOP-lowering efficacy of PF-tafluprost in patients (n=158) who had exhibited ocular surface side effects with a BAC-preserved latanoprost regimen. What they found was that PF-tafluprost maintained IOP at the same level as latanoprost, but was better tolerated and resulted in increased patient satisfaction, drop comfort and quality of life. One of the reasons why BAC can cause ocular surface disorders is through goblet cell loss. Goblet cells are the main source of ocular surface mucoproteins and play a central role in tear film stability (9). They have been shown to diminish in number following exposure to BAC-containing topical glaucoma therapies (10), and it’s thought that leads to decreased mucin production, tear film instability and ultimately, in many cases, OSD (11). Switching to PF-eyedrops can help improve goblet cell count, but in treatment-naïve patients with glaucoma, why start with PC therapy at all?
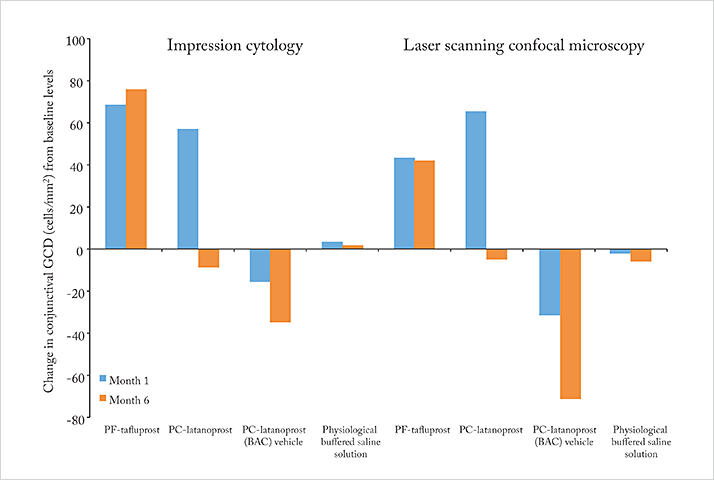
Mastropasqua et al. (12) assessed the goblet cell density (GCD) in 30 eyes of treatment-naïve patients with primary OAG with both in vivo laser scanning confocal microscopy and impression cytology. Patients were randomized to receive either PF-tafluprost, BAC-preserved latanoprost, the BAC-containing latanoprost vehicle or physiological buffered saline solution, and GCD assessments were performed at months 1 and 6 (Figure 1). What was clear was that BAC was bad news for GCD – relative to baseline levels, the numbers were reduced at both timepoints. Notably, PC-latanoprost and PF-tafluprost application actually increased GCD by month 1, but by month 6, eyes that received preserved latanoprost had GCD that was lower than baseline levels, whereas PF-tafluprost-receiving eyes maintained their increased levels of GCD. The study authors confirmed the earlier suggestion by Pisella et al. in 2004 (13) that prostaglandin analogs might have a protective or beneficial effect on goblet cells – and that prolonged BAC exposure can lead to toxicity that masks the beneficial effects of these drugs. Given the evidence that the preservatives present in many topical glaucoma medications have no efficacy benefits, but can cause OSD, reduce regimen adherence and, by increasing side effects risk, speed the progression of glaucoma (4), the question is: why persist with preservatives, when preservative-free therapies are available?
Next month
As a significant proportion of patients with glaucoma are sensitive to the preservatives contained in many topical glaucoma therapies, it’s therefore important to diagnose these patients. We’ll review the diagnostic tools and the European Glaucoma Society’s guidelines for identifying these patients.References
- EW Leung, et al., “Prevalence of ocular surface disease in glaucoma patients”, J Glaucoma, 17, 350–355 (2008). PMID: 18703943. C Erb, et al.,“German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye”, Graefes Arch Clin Exp Ophthalmol, 246, 1593–601 (2008). PMID: 18648841. A Camp, et al., “Dry eye specific quality of life in veterans using glaucoma drops”, Cont Lens Anterior Eye, 38, 220–225 (2015). PMID: 25737401. P Denis, et al., “Medical outcomes of glaucoma therapy from a nationwide representative survey”, Clin Drug Investig, 24, 343–352 (2004). PMID: 17516721. M Irkec, et al., “Are preservatives necessary to improve efficacy of some glaucoma drops?”, Br J Ophthalmol, 97, 1493–1494 (2013). PMID: 24216677. T Hamacher, et al., “Efficacy and safety levels of preserved and preservative-free tafluprost are equivalent in patients with glaucoma or ocular hypertension: results from a pharmacodynamics analysis”, Acta Ophthalmol Suppl, 242, 14–19 (2008). PMID: 18752510. A Shedden, et al., “Comparison of the efficacy and tolerability of preservative-free and preservative-containing formulations of the dorzolamide/timolol fixed combination (COSOPTTM) in patients with elevated intraocular pressure in a randomized clinical trial”, Graefes Arch Clin Exp Ophthalmol, 248, 1757–1764 (2010). PMID: 20437244 H Uusitalo, et al., “Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication”, Acta Ophthalmol, 88, 329–336 (2010). PMID: 20546237. MJ Doughty, JP Bergmanson, “New insights into the surface cells and glands of the conjunctiva and their relevance to the tear film”, Optometry, 74, 485–500 (2003). PMID: 12926821. MY Kahook, R Noecker, “Quantitative analysis of conjunctival goblet cells after chronic application of topical drops”, Adv Ther, 25, 743–751 (2008). PMID: 18670744. G Van Setten et al., “Severe Dry Eye Disease – Facing the Treatment Challenges”, European Ophthalmic Review, 8, 87–92 (2014). G Van Setten, et al., “Conjunctival goblet cells density and preservative-free tafluprost therapy for glaucoma: an in vivo confocal microscopy and impression cytology study”, Acta Ophthalmol, 91, e397–405 (2013). PMID: 23601909. PJ Pisella, et al., “Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study”, Invest Ophthalmol Vis Sci, 45, 1360–1368 (2004). PMID: 15111589.
