- Modern risk factors are creating dry eye in younger patients
- Optimizing the ocular surface to eliminate inflammation increases positive outcomes
- Nutraceuticals and healthy diet can regenerate the ocular surface naturally
- The modern epidemic of dry eye requires proactive care
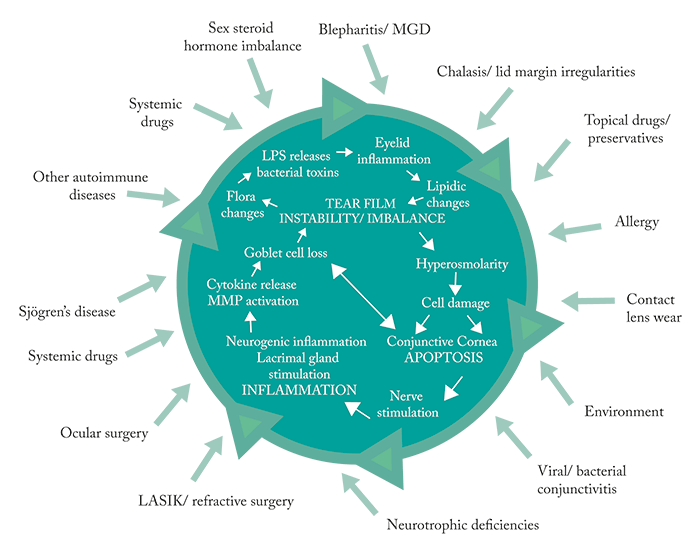
Even though we now have a greater knowledge of the etiology of ocular surface disease (OSD), as well as better diagnostics and a growing array of therapeutics than ever before, there still seems to be a tendency to try to pigeonhole this disease into one of two specific categories – evaporative dry eye or aqueous-deficient dry eye. We need to stop trying to post these problems into one box or the other. We need to start thinking about the patient. Ask: what symptoms are present? What risk factors are creating or exacerbating the problem? The patient’s medical history, medications, habits, profession, diet, and lifestyle will all affect what happens on the ocular surface. Ultimately, it doesn’t matter if the patient’s particular case of dry eye is evaporative or aqueous-deficient, or if everything in combination is leading to the breakdown of the lacrimal function unit. The stressor leads to dysfunction and imbalance of tear film, which creates inflammation and perpetuates this cycle (Figure 1) – and that’s what needs to be addressed. If the stressors can be reduced or eliminated, the cycle can be controlled, and in certain cases, broken.
Screen burn
The prevalence of OSD is growing at an alarming rate, and it’s affecting increasingly younger people. Why? One of the greatest stressors in today’s society: screen time. Twenty years ago, this wasn’t an issue for the majority of the population – but interacting with a screen (computer monitor, tablet or phone) is near-ubiquitous today. The use of digital devices puts children at great risk of developing OSD (1) – a risk that only increases with age. American teens typically spend an average of nine hours a day consuming digital media (2) in addition to their school and homework that may also require screen time. Screen time reduces blink rate (3)(4) – by as much as 60 percent during computer use (5). Up until a few years ago, everyone thought about dry eye in terms of aqueous-deficient or evaporative without viewing dry eye disease (DED) as part of ocular surface disease (OSD). What it boils down to is: no matter the cause of the dry eye, there must be a balance. Tear film has to have all the necessary components to do its job correctly. It’s a supply and demand issue. Patients may not have an autoimmune issue, such as Sjögren’s syndrome, but if they are staring at screens all day and not blinking, it does become a big risk factor that can, by itself, lead to debilitating damage to the ocular surface. With reduced blink rate comes greater meibomian gland congestion and worsening tear film break up times (TBUT), which can lead to meibomian gland disease (MGD). Additional factors like systemic co-morbidities, contact lens use, cosmetics, cosmetic surgery (such as eyeliner tattoos – which destroy meibomian glands), and medications that cause dry eye (like antihistamines) can all cause OSD. Irrespective of the etiology, what this means is that inflammation is introduced into the picture – and starts the OSD ball rolling.Deeper into dry eye
A greater understanding of inflammation has been pivotal in triggering essential research into the progression of dry eye. Researcher physicians, such as Stephen Pflugfelder, have spent countless years looking at the markers, mediators, and inflammatory cascade elements that exist in acute or chronic stages of dry eye. As a result, we now have a more qualitative, hard evidence-based approach. Even as recently as 2007, the Tear Film and Ocular Surface Society (TFOS)’ Dry Eye Workshop (DEWS) report failed to talk about signs alone as being enough to diagnose dry eye. We’d always been taught to give more credence and weight to staining and would dismiss the diagnosis of dry eye if the patient complained but had no staining. Now, we have a greater understanding, particularly in those patients where such a disconnect existed – typically, younger patients that were very symptomatic but didn’t stain, or much older patients who stained remarkably, even to the point of epithelial defects, but were completely asymptomatic. Now, we look at the different components of a more detailed clinical exam that includes more than going straight to the cornea with fluorescein staining. We understand the prevalence of MGD and are more acutely aware of it during exams. We can now test for the presence of inflammation with matrix metalloprotease (MMP-9) with InflammaDry (Quidel Corporation), perform imaging of the meibomian glands, and gather comprehensive information on the patient through the use of the Ocular Surface Disease Index (OSDI) or Standard Patient Evaluation of Eye Dryness (SPEED) questionnaires. Tear osmolarity is my go-to diagnostic for all of my patients because of the information it provides. If the test outcome is positive in the range of moderate to severe OSD, or even possibly in the normal range but the patient displays clear evidence of OSD, I now have objective evidence that points me in a better direction to build a treatment plan.Treatment paradigms
Compiling patient-specific information is a good way to determine the best and most effective therapies for that patient. Patients with less severe cases can start out slowly, with more home-based remedies, while more advanced cases will need more rigorous therapies right from the start. The educational process should also not be overlooked: patients need to understand what actors are in play, so that they can be proactive in preventing further damage, like being cognizant of their blinking habits and practicing a full blink. It’s amazing how little things can make a big difference!Artificial tears
Previous generations didn’t have a great deal of dry eye treatments at their disposal other than perhaps carboxymethylcellulose artificial tears. Artificial tears are palliative and can be a good starting point for patients who want to start with something that is not a prescription medication. Indeed, the artificial tears that we have available today are improved from past formulas, with various active ingredients, such as hyaluronic acid, and a wide range of different viscosities. Preservative-free options keep tears from exacerbating OSD symptoms and we now have customized tears that can treat lipid deficiency. For patients with occasional symptoms, artificial tears may be sufficient. If patients need the artificial tears daily or multiple times a day, then they are not adequately managing their dryness and you will discuss further therapy.Nutraceuticals
As with artificial tears, past generations didn’t have access to scientifically proven nutraceuticals, and they didn’t really understand the need for them. We have, more recently, begun to truly understand the etiology behind dry eye and the connection with diet and nutrition. The average American’s diet is full of pro-inflammatory molecules that can not only exacerbate systemic conditions in an inflammatory context like cardiovascular disease (6), but also influence conditions, such as dry eye. Who would have thought that a diet overly rich in meat and dairy could make our eyes worse? But what we eat does matter. I try to push a healthy diet and nutraceuticals from the beginning. The last thing any patient wants is to be on prescriptions for the rest of his or her life. Adding nutraceuticals from the very beginning is an excellent course of action as it will help with any stage of the disease. Patients appreciate using a nutraceutical that truly has an anti-inflammatory effect and aids in not only rebuilding different components of tear film, but also benefits lids and the way the meibomian glands function, as well as the clarity of meibum that is being egressed and produced (7). We are very fortunate today to have more than one anti-inflammatory nutraceutical on the market, particularly advanced omegas like HydroEye (ScienceBased Health), which will improve and regenerate the ocular surface in a more natural way.We’re now starting to learn about the right combinations of fatty acids to truly improve MGD, like the omega-3s eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) that are commonly found in fish oil, but also gamma-linolenic acid (GLA). While people have been taking omega-3s for some years to improve a variety of conditions (6) (8)(9)(10)(11), omega-6 fatty acids were typically thought to be uniformly bad. However, GLA is an anti-inflammatory omega-6 fatty acid that has been shown in studies to be very effective in combating dry eye (12). The problem is that GLA is only found in plants, such as evening primrose, or borage seed oil and blackcurrant seed oil – not foods commonly consumed by humans (at least not in quantities large enough to make a difference). In order to reap the benefits in dry eye, we must turn to nutraceuticals that combine EPA with GLA to suppress pro-inflammatory mediators while stimulating anti-inflammatories (7)(13)(14)(15)(16).Upping the ante
While diet and supplements can be very effective for those with earlier disease and are of benefit to all patients, more advanced technology is now available for when further measures are needed. Daily warm compresses can be helpful but LipiFlow treatments are more useful from a compliance and quality of life standpoint; a single treatment is much easier and more effective than a daily compress regimen that may not always be followed properly. I will also prescribe other targeted therapies, such as thermal pulsation, as necessary. Another promising new development is neurostimulation with Oculeve TruTear (Allergan), an intranasal neurostimulator that is inserted into the nasal passage to stimulate the trigeminal nerve, which results in tear production. While more testing is needed, it has been shown to be effective in improving ocular comfort and staining scores (17), as well as increasing the mucin layer and aqueous layer of tear film (18)(19). Punctal occlusion is another option, although if the patient has a more meibomian gland-based dry eye (particularly if inflammation is present), then I will not use plugs; keeping an inflamed tear on the eye will only cause more damage. However, once the eye is quiet, punctal occlusion can be beneficial.In the pipeline
Several companies are now delving into amniotic cytokine treatment processes. In my experience, these treatments have been phenomenal – and I’ve even seen significant improvement in as short as a month. Treatments are also available for patients with filamentary keratitis exacerbated with blepherospasms. Beginning treatment with Botox injections to control the spasms is of more benefit than starting with anti-inflammatories right away, as those can take six to twelve weeks to show efficacy. One new therapy in development is Tavilermide (Mimetogen/Allergan) which induces the natural anti-inflammatory protein, mucin – and there are dozens of different topical medications, including different formulations of cyclosporine 0.1% (Sun Pharmaceuticals), other novel anti-inflammatories, and potentially mucin-producing mimetics that enhance the natural tear film, that will continue to expand topical options for patients. There are also unique thermal meibomian gland interventions that are being devised and in clinical trials to support MGD treatments.Prepping the Ocular Surface for Surgery
When preparing patients for cataract surgery, accurate diagnostics are first and foremost. Topography and meibography are key images. Infrared meibography (Lipiscan, TearScience/JJV) provides me an instantaneous snapshot of the presence of disease, severity level and gives a sense of chronicity of the ocular surface disease process. Not all patients will have gland drop out, but if they do, it alerts me to the patient’s higher risk status and allows me to more appropriately prepare the patient. If the patient has dry eye disease with ocular surface staining, we have a discussion on the presence of OSD, and the treatment options – both acute in preparing the ocular surface for cataract surgery, as well as for chronic maintenance. To rapidly stabilize the ocular surface, a short three-week taper of a topical steroid alongside frequent preservative-free artificial lubrications can quickly improve the cornea to facilitate accurate cataract diagnostics. I prefer a preservative-free dexamethasone, or loteprednol ointment, particularly if the patient is on other topical medications (glaucoma agents) or has a known hypersensitivity to preservatives. I also advocate for an oral nutraceutical and blepharitis management, and schedule a return appointment in 3–4 weeks for repeat measurements. However, if we are looking for a specific outcome of prolonged improved uncorrected visual acuity after surgery, the patient will likely need to commit to using some medication to control the chronic dry eye disease, and this may be with a daily supplement or daily prescription anti-inflammatory drop(s) to maintain his or her quality of vision. If the dry eye disease is more reticent and mild, I will be more reserved. With mild dry eye disease, patients will not have staining, but their OSD can certainly worsen post-operatively, thus patient education is important. These are patients for which oral omegas and palliative artificial lubrication can work effectively. Those with recalcitrant, more severe dry eye whose corneas do not improve with aggressive lubrication and a short course of topical steroids will undoubtedly need greater therapy, management – and handholding. This patient will unlikely be an appropriate candidate for an extended depth-of-focus or multifocal presbyopia-correcting lens. For patients who have central staining and issues with blinking due to co-morbidities, such as Parkinson’s, I recommend using PROKERA (Bio-Tissue) sutureless biologic corneal bandage. The cryopreserved amniotic membrane is placed beneath the upper and lower lids and is very effective in advancing corneal healing, reducing inflammation, and optimizing the ocular surface for a variety of indications, including severe dry eye in patients who haven’t responded to other treatments. PROKERA is placed for 5–6 days then removed at the follow-up visit. Diagnostic measurements for cataract surgery can be performed within 24–48 after the treatment has been completed.A call to action
Knowing that modern life’s increased risk factors are going to lead to an epidemic of severe dry eye patients earlier in life with recalcitrant disease, we need to be as proactive as possible. Education and the use of nutraceuticals and a healthy diet is always a good place to start and are beneficial for all patients no matter their level of disease. Dry eye can severely affect patients’ quality of life, and improper diagnosis and treatment only leads to more quality of life issues and difficult to manage patients. Taking care of patients earlier with better diagnosis and therapy is ultimately to our benefit as well as the patients’, and allows us to continue to protect them into the future. Elizabeth Yeu is a board-certified ophthalmologist in Cornea, Anterior Segment & Refractive Surgery at Virginia Eye Consultants. She actively serves as an examiner for the American Board of Ophthalmology and is a Governing Board member and Chair of the Young Eye Surgeons Committee of the American Society of Cataract and Refractive Surgery (ASCRS). Disclosure: Elizabeth consults for Alcon, Allergan, AMO, Bausch + Lomb, Shire, TearScience, and TearLab.References
- JH Moon et al., “Association between video display terminal use and dry eye disease in school children”, J Pediatr Ophthalmol Strabismus, 51, 87–92 (2014). PMID: 24495620. M Rosenfield , “Computer vision syndrome: a review of ocular causes and potential treatments”, Ophthalmic and Physiol Opt, 31, 502–15 (2011). PMID: 21480937. M Acosta et al., “The influence of eye solutions on blinking and ocular comfort at rest and during work at video display terminals”, Experimental Eye Research, 68, 663–9 (1999). PMID: 10375429. S Patel et al., “Effect of visual display unit use on blink rate and tear stability”, Optom Vis Sci, 68, 888–892 (1991). PMID: 1766652. C Blehm et al., “Computer vision syndrome: A review”, Surv Ophthalmol, 50, 253–262 (2005). PMID: 15850814. D Giugliano et al., “The effects of diet on inflammation”, J Am Coll Cardiol, 48, 677–685 (2006). PMID: 16904534. JD Sheppard et al., “Long-term supplementation with n-6 and n-3 PUFAs improves moderate-to-severe keratoconjunctivitis sicca: a randomized double-blind clinical trial”, Cornea, 32, 1297–1304 (2013). PMID: 23884332. JX Kang et al., “Prevention of fatal arrhythmias by polyunsaturated fatty acids”, Am J Clin Nutr, 71, 202S–207S (2000). PMID: 10617972. WE Connor , “Importance of n-3 fatty acids in health and disease”, Am J Clin Nutr, 71, 171S–175S (2000). PMID: 10617967. E Lopez-Garcia et al., “Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women”, J Nutr, 134, 1806–1811 (2004). PMID: 15226473. A Zampelas et al., “Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study”, J Am Coll Cardiol, 46, 120–124 (2005). PMID: 15992645. S Barabino et al., “Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component”, Cornea, 22, 97–101 (2003). PMID: 12605039. C Creuzot et al., “Improvement of dry eye symptoms with polyunsaturated fatty acids [in French]”, J Fr Ophtalmol, 29, 868–873 (2006). PMID: 17075501. F Brignole-Baudouin et al., “A multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patients”, Acta Ophthalmol, 89, e591–e597 (2011). PMID: 21834921. JB Barham et al., “Addition of eicosapentaenoic acid to gamma-linolenic acid-supplemented diets prevents serum arachidonic acid accumulation in humans”, J Nutr, 130, 1925–1931 (2000). PMID: 10917903. S Viau et al., “Polyunsaturated fatty acids induce modification in the lipid composition and the prostaglandin production of the conjunctival epithelium cells”, Graefes Arch Clin Exp Ophthalmol, 250, 211–222 (2012). PMID: 21894532. NJ Friedman et al., “A nonrandomized, open-label study to evaluate the effect of nasal stimulation on tear production in subjects with dry eye disease”, Clin Ophthalmol, 10, 795–804 (2016). PMID: 27217719. K Gumus et al., “Randomized, controlled, crossover trial comparing the impact of sham or intranasal neurostimulation on conjunctival goblet cell degranulation”, Am J Ophthalmol, 177, 159–168 (2017). PMID: 28302532. K Gumus et al., “Intranasal tear neurostimulation: an emerging concept in the treatment of dry eye”, Internatl Ophthal Clin, 57, 101–108 (2017). PMID: 28282317. C Baudouin, “A new approach for better comprehension of diseases of the ocular surface”, J Fr Ophtalmol, 30, 239–46 (2007). PMID: 17417148.
