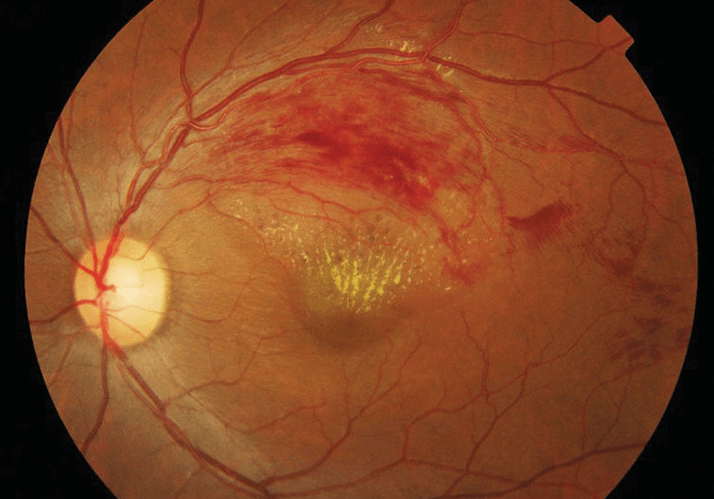
- Retinal vein occlusion – particularly ischemic RVO – commonly leads to macular edema
- Intravitreal steroid implants and anti-VEGF therapies are milestone improvements in treatment
- The timing of therapeutic intervention has not yet been optimized
- Fixed dosing schedules should give way to interventions based on visual assessments and other disease state markers
Retinal vein occlusion (RVO) affects 16 million people worldwide, usually the middle-aged or older. It is caused by thrombosis, the clogging of the retinal veins, which may occur in the four branch veins that drain blood from the capillaries of the retina – branch vein retinal occlusion (BVRO; Figure 1) – or in the central retinal vein formed by the combination of the four branch veins – central retinal vein occlusion (CRVO). Occlusion may be either ischemic or non-ischemic in nature, with the former having considerably poorer prognosis. Left untreated (or if treatment fails), persistent RVO can lead to macular edema (ME), the swelling or thickening of the macula caused by leaking blood vessels. ME is the primary cause of RVO-related blindness, neovascularization, and retinal detachment. Longer durations of RVO-related ME are associated with poorer outcomes.
Until fairly recently, there was little that could be done for a patient that developed ME after an RVO episode. The standard care was grid laser photocoagulation for ME in non-ischemic BRVO (1) and observation of macular edema in the case of CRVO (2). Today, it’s a different story: implantable and injectable drug-based options are available, notably a dexamethasone intravitreal implant (Allergan’s Ozurdex) and inhibitors of vascular endothelial growth factor (VEGF), including an antibody (Genentech’s Lucentis [ranibizumab]) and a fusion protein (Bayer/Regeneron’s Eylea [aflibercept]). Recently, pars plana vitrectomy has also been shown to be effective for improving macular thickness, volume, and sensitivity in patients with macular edema secondary to ischemic CRVO (3). Here I will cover the current state of the art, issues arising, and possible solutions to them.
Dexamethasone is a potent, water-soluble corticosteroid with many therapeutic indications, mostly anti-inflammatory. As the sclerotic membrane of the eye hinders the egress of intravitreally injected drugs, this means that the steroids introduced into the eye act mainly on the eye – to great effect. The intravitreal dexamethasone-containing implant (Ozurdex; Figure 2), which was approved by the US Food and Drug Administration (FDA) in 2009, comprises micronized dexamethasone within a biodegradable copolymer of lactic and glycolic acids. The implant is inserted into the eye through a small pars plana puncture using a customized applicator system and, once in situ, it is releases dexamethasone over a period of months.
Phase III clinical trials (4) demonstrated efficacy and safety of the dexamethasone intravitreal implant for the treatment of patients with ME due to CRVO or BRVO. Ozurdex is most likely to improve vision in patients who have experienced RVO-related ME of a relatively short duration. While treatment initiated up to three months after the onset of disease is still somewhat efficacious, further delay diminishes impact. The implant also appears to be effective in vitrectomized eyes with ME secondary to CRVO and in those with chronic edema from BRVO. However, early retreatment after 16 weeks (instead of 24 weeks) was required in 50% of CRVO and BRVO cases in order to maintain the improved functional and anatomical results. Analyses of the initial and follow-on studies have suggested that the original treatment protocols could have been better (5-6%). It is not clear that Ozurdex implants remained effective for six months, the schedule employed in the original study, and authors of subsequent studies have suggested the following. Reinjection at shorter intervals; as-needed retreatment protocols (particularly in CRVO); and combination treatment. Anti-VEGF agents, the first of which was introduced in 2005, revolutionized the treatment of wet age-related macular degeneration (AMD). Their use in treatment of ME secondary to RVO has been more recent, but equally impactful. Tissue hypoxia due to primary vascular occlusive disease is the most common driver of VEGF synthesis; as RVO is associated with increased VEGF expression levels, the use of VEGF inhibitor drugs would, in theory, improve matters in RVO-related ME.
Ranibizumab, a monoclonal antibody fragment that binds VEGF-A, was approved by FDA in 2010 for the treatment of ME secondary to both BRVO and CRVO; European Medicines Agency (EMA) approval followed in 2011. Ranibizumab is effective over a 12-month period, but beyond this, patients with CRVO may require treatment administration more frequently than every three months, the current standard. Less frequent follow-up and fewer ranibizumab injections in the second year of treatment were associated with a decline in vision in CRVO patients, although stable vision was achieved in patients with BRVO. Aflibercept is a protein engineered to inhibit both VEGF and a related angiogenic molecule, placental growth factor (PGF). The FDA approved aflibercept in September 2012 for the treatment of ME resulting from CRVO, and a regulatory submission has been filed with the EMA. Data from two ongoing Phase III studies of aflibercept in patients with CRVO (7–10) indicate that intravitreal injection of aflibercept improves visual acuity and central retinal thickness, eliminates progression resulting from neovascularization and is associated with a low rate of treatment-related ocular adverse events. Although efficacy starts to wane after 12 months of treatment, taken together, the results are encouraging for patients with CRVO and the longer duration of action of aflibercept in comparison with ranibizumab makes it an appealing option (11).
Each of the above therapy options has associated safety issues. Ozurdex use can raise intraocular pressure (IOP). Clinical trial data have shown that 16 to 27 percent of Ozurdex-implanted subjects developed significantly raised IOP, with some requiring therapy to manage this, including one percent of patients who needed surgical intervention (4–6). In addition, 26 percent of patients who receive two Ozurdex implants formed cataracts; the rate was five percent in control patients. Postmarketing surveillance has also revealed that dexamethasone implants can become discolored, or even cause endophthalmitis. For ranibizumab and aflibercept a range of safety concerns have been raised. There are serious but rare (fewer than one in a thousand injections) ocular adverse events relating to the intravitreal injection procedure, including endophthalmitis, rhegmatogenous retinal detachments, and iatrogenic traumatic cataracts. Side effects associated with the active agents include hemorrhage, eye pain, maculopathy, retinal exudates, increased intraocular pressure, optic disc vascular disorder, and retinal vascular disorder. Chronic use brings the risk of adverse events developing over a longer time-period, and tolerance and tachyphylaxis start to become issues (12). There is also a risk of cardiovascular side effects – atrial fibrillation and peripheral edema occur in up to 5 and 6 percent of patients receiving ranibizumab, respectively – and the potential for arterial thromboembolic events (ATEs), such as stroke, myocardial infarction, and vascular death. Finally, renal failure (up to 7 percent) and chronic renal failure (up to 6 percent) have also been reported. There are economic and cost-effectiveness considerations to throw into the mix too. The introduction of anti-VEGF therapy for RVO has led to substantial increases in clinical workload – both in terms of carrying out the implant procedures and in ensuring that patients have adequate follow-up appointments. The introduction of anti-VEGF treatments for CRVO could further exacerbate pressure on clinic capacity in the hospital eye service.
One way to reduce clinical workload and the impact on resources is to determine a patient's best treatment regimen based upon their disease characteristics. Vision loss, visual acuity (VA) instability, or other signs of an active disease state could be used as markers for when an individual requires treatment, rather than using fixed dosing schedules. This should match patients to their best therapeutic option, reduce over- and under-treatment, minimize unnecessary drug exposure and optimize the benefit-risk profile of these interventions. Today, there is also a focus on improving the efficacy and safety of these therapies by, for example, optimizing retinal bioavailability, which is hampered by the blood-retina barrier. Nanoparticle formulations combined with new delivery systems promise increased drug penetration at the target site, reduced dose required to achieve therapeutic effects, prolonged effects and reductions in toxicity and systemic side-effects (13). Integrin peptides are a new treatment option currently under investigation. Integrins are transmembrane receptors involved in cell adhesion, signal transduction, cell proliferation, shape and motility; in the eye, multiple integrins are involved with angiogenesis. Allegro Ophthalmics have a small peptide (ALG-1001) that inhibits multiple integrin receptor sites, that is currently undergoing clinical evaluation for vascular eye diseases, including macular edema due to retinal vein occlusion. In preclinical studies, ALG-1001 has shown promise as a monotherapy and when combined with ranibizumab. Further studies are ongoing (14). Dexamethasone intravitreal implants and anti-VEGF angiogenesis inhibitors have dramatically improved the management of ME following retinal vein occlusion. Yet unmet needs remain: drugs that are less invasive in administration, more effective over longer periods, and have better safety profiles are required. I am confident that we will have them, and soon. Marianne L. Shahsuvaryan is Professor of Ophthalmology at Yerevan State Medical University, Yerevan, Armenia.
