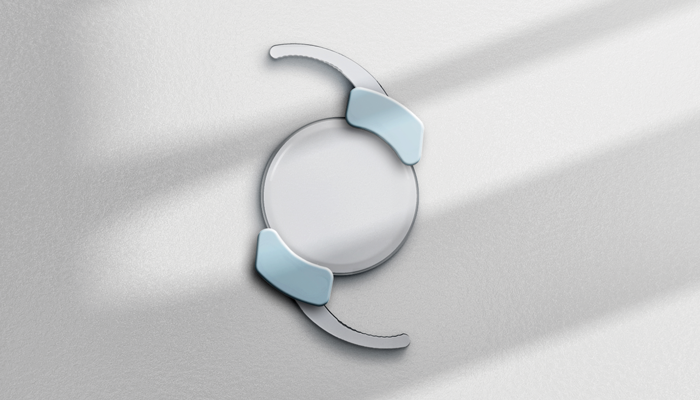
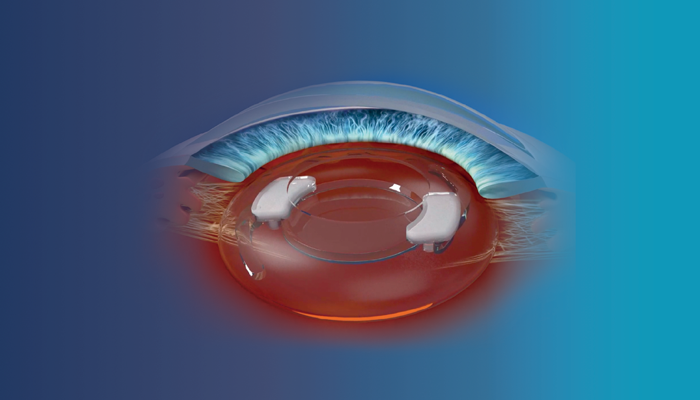
SpyGlass Pharma is aiming to revolutionize the field of glaucoma treatment with the development of its new, innovative drug delivery platform. Working by securing to the haptics of an intraocular lens (IOL), the SpyGlass platform is implanted into the capsular bag, with the IOL, at the time of routine cataract surgery. The initial product is designed to continuously elute bimatoprost therapy for multiple years to lower intraocular pressure (IOP) in patients with glaucoma or ocular hypertension (OHT).
CEO of SpyGlass Pharma, Patrick Mooney, says that although MIGS procedures are a great option for many patients with glaucoma, research suggests that 75 percent of cataract surgeons are not routinely performing MIGS procedures at the time of cataract surgery (1). In addition, claims data indicates that one in five cataract procedures in the United States today are done in patients with open-angle glaucoma or OHT (2). “By implanting at the time of cataract surgery, surgeons can continue using their tried and tested techniques without having to learn and adopt new skills,” says Mooney.
SpyGlass’ elegant technology not only benefits the surgeons conducting the procedure, but also the patient. Despite current therapeutics being effective at lowering IOP, medicines cannot work when patients don’t take them. SpyGlass technology resolves the problem of patient non-adherence by delivering prostaglandin therapy consistently and continuously each day. As a result of bimatoprost being delivered from within the eye, the cumulative daily release needed to achieve a therapeutic effect is significantly lower than a single topical drop of bimatoprost, potentially making it a more effective treatment option for patients with glaucoma.
The clinical efficacy of the SpyGlass technology has been confirmed in its first-in-human (FIH) feasibility trial. In this trial, researchers enrolled 23 subjects who had a visually significant cataract and glaucoma. After being washed out of their topical medications and having their baseline IOP recorded, researchers randomized patients to receive cataract surgery and one of three doses of bimatoprost, via the SpyGlass drug delivery platform. Over a nine-month period, the trial found that, across all three doses, mean IOP-lowering was 45 percent from baseline. The visual results were also excellent and in line with best-in-class IOLs on the market today. Mooney confirms that SpyGlass is now proceeding with their US phase I/II clinical trial that will test multiple doses of bimatoprost therapy in the SpyGlass system vs. control, marking a significant milestone for the company.
“The development of SpyGlass would not be possible without amazing team members with deep history and understanding of ophthalmic pharmaceuticals and medical devices,” adds Mooney. SpyGlass was co-founded by Dr. Malik Y. Kahook and Glenn Sussman, both of whom are serial inventors and entrepreneurs with over 50 years of combined experience in the ophthalmic space. Together, Malik and Glenn spearheaded the innovation in ClarVista Medical which developed a modular IOL technology that was acquired by Alcon in 2017. SpyGlass’ COO, James Dennewill, has over 20 years of industry experience and is a specialist in the development of products, people, and processes required to bring new technologies to market. Spyglass’ Vice President of Regulatory Affairs, Nina Nguyen, also plays a key role in the company, bringing over 20 years of global regulatory affairs leadership in both the pharmaceutical and medical device industry, with relevant experience at Glaukos and Allergan. Mooney joined the company in 2021 as CEO and adds to the team’s expertise with his experience of eye care that spans the surgical, vision-care and pharmaceutical spectrum, and which includes product launches in retina, glaucoma, dry eye as well as medical devices. Most recently, Mooney was Vice President and Head of the Ophthalmology Franchise at Novartis where he was responsible for the overall strategy and growth of US ophthalmic pharmaceuticals.
The wider team at SpyGlass, consisting of engineers, scientists, and an experienced clinical and regulatory team, are no less important. All have exhibited a proven track record of both innovating and commercializing key products that improve the standard of care for patients and surgeons.
In addition to addressing glaucoma patients at the time of cataract surgery, the SpyGlass system can also effectively elute both steroids and nonsteroidal anti-inflammatory drugs, used to address pain and inflammation post cataract surgery. Mooney says, “as SpyGlass technology allows for adjustments in daily elution and total payload, the team also sees an opportunity to address other chronic conditions such as uveitis and age-related macular degeneration.”
“The SpyGlass drug delivery platform undoubtedly introduces an innovative and promising treatment option for glaucoma patients, and has the potential to establish itself as the gold standard of glaucoma treatment in the years to come.”
References
- CMS claims data (2022)
- MarketScope, “2022 Glaucoma Surgical Device Market Report” (2022).
