- Assessing patients’ postoperative refraction after cataract surgery and monofocal IOL implantation was relatively simple
- This is not the case with premium IOLs – their advanced optics can catch you out
- Both subjective and objective refraction assessments have to be performed with great care and with modified protocols in order avoid surprises
- Here, I present a number of tips to ensure that you can optimally assess patients’ refraction
In some respects, cataract surgery was simpler in the days before premium intraocular lenses (IOLs) – i.e. toric, multifocal, extended range of vision and accommodative IOLs. In the old days, straightforward cataract surgery involved simply replacing your patients’ cloudy crystalline lens with a standard monofocal spherical IOL. Really, the big refractive challenge was to ensure that the lens was well-centered, in order to avoid unwanted prismatic effects and minimize the visual field constriction that’s inherent to high positive lenses. But once the sutures were removed, measuring postoperative refraction was, on the whole, simplicity itself.
Early monofocal spherical IOL models induced significant amounts of spherical aberration – but this was at a time when cataract procedures were performed later in life, on cloudier lenses, with no better alternatives. Patients therefore had a higher tolerance to residual refractive errors: the visual improvements were to them, dramatic. The introduction of aspheric IOLs improved matters (and patients’ vision) as they reduced higher-order aberrations, but even then, patients still accepted that they might need to use refractive correction – typically spectacles – for specific tasks after cataract surgery such as reading or driving. This scenario has changed drastically over recent years. Improvements in surgical techniques, smaller incision sizes, the availability of toric, multifocal, extended range of vision and accommodative IOLs, and the optimization of corneal biometric measurements with advanced devices, have enabled us to greatly improve visual outcomes. The consequence is raised expectations. Emmetropia, across all distances, is the objective – and it’s bad news if a patient expecting spectacle-free vision after the procedure doesn’t achieve it.
Pre- and peri-operative considerations
One of the biggest problems with using today’s sophisticated, premium IOLs is predicting the postoperative axial position of the IOL optic – the “Effective Lens Position” (ELP) – which determines the power of the lens that you need to implant. The formulas used to calculate it are right only around 80 percent of the time. The capsulorhexis is crucially important for optimal postoperative outcomes too – large rhexes combined with capsular bag contraction can shift the IOL anteriorly (with a myopic shift); if the rhexis is small, phimosis may result, with posterior displacement of the IOL and a hyperopic shift. What you want is a rhexis that’s just smaller than the optic, so that the capsular bag will “shrink wrap” around the optic and line up with the plane of the zonules. Finally, the IOL geometry does not replicate the anatomy of the crystalline lens, and as every person’s cornea has natural structural – and therefore optical – aberrations, that will prevent optical perfection.Reconsidering refraction in modern IOLs
Refractive, diffractive, refractive-diffractive, apodized, echelette… there are a number of different optical principles that underpin today’s range of premium IOLs. Because of this, practitioners have to reconsider whether their methods of measuring refraction are still appropriate… or might need a little refinement. Using the right refraction protocol after the implantation of multifocal and extended range of vision IOLs is extremely important, as their defocus tolerance is far lower than with monofocal IOLs. The effect of small amounts of residual refractive errors on visual acuity (VA) is quite significant in eyes implanted with multifocal and related IOLs (1). Part of the problem is that it’s not trivial (and sometimes impossible) to correct these refractive errors once the multifocal IOL has been implanted. You really do not want to find yourself in the position of having to give the patient limbal relaxing incision or placing them under an excimer laser to try to correct their postoperative residual refraction (2).This article aims to discuss how to measure VA to understand the behavior of various models of IOLs at different distances. We’ll look at the reliability of retinoscopy, autorefraction, Jackson’s cross cylinders (JCCs), and subjective refraction, and examine what complications can occur after implantation of a multifocal or extended range of vision implant that may affect VA and refraction – such as decentration or posterior capsular opacification (PCO).
Defocus curves
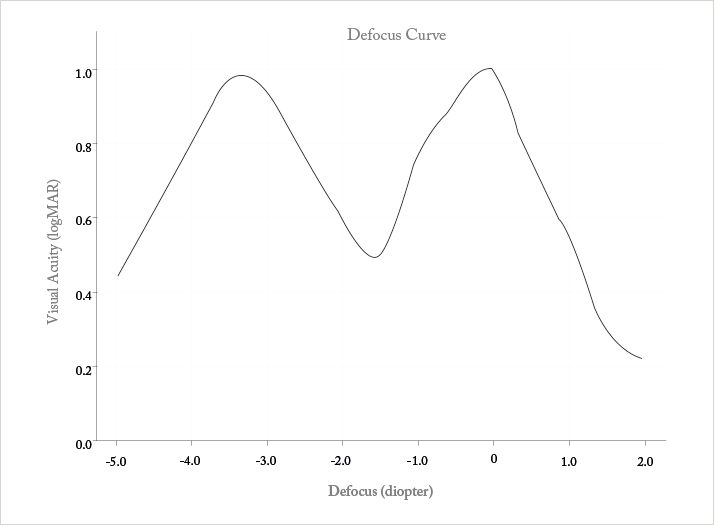
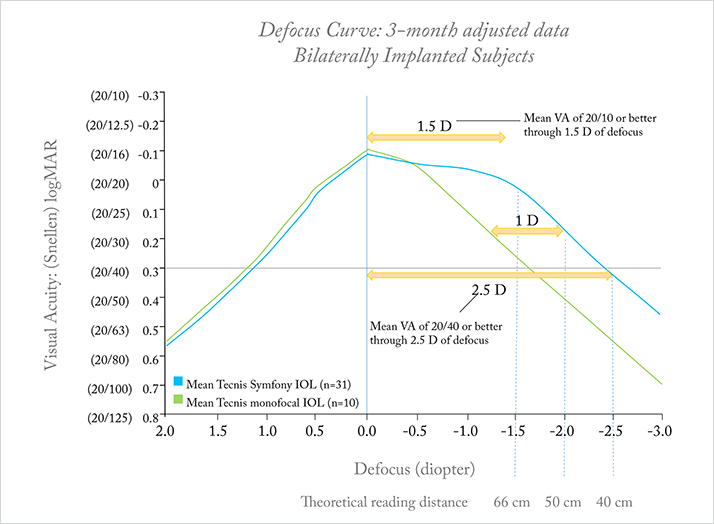
The first step in assessing the refractive behavior of a premium IOL is to determine what type of IOL has been implanted. If there’s any doubt at all over this, the patient will need to undergo a comprehensive slit lamp examination that will let the examiner figure out if the IOL is accommodative, refractive (segmented or with circular sectors), or diffractive (with concentric rings) in design. The accepted method of evaluating VA at different distances in patients with IOLs is the defocus curve – a fairly simple process. Under constant shaded illumination, and using conventional optotypes, the patient is asked to view a distant eye chart, and is at first monocularly corrected and then binocularly corrected for their best distance acuity; this sets a zero benchmark for all subsequent lenses tested. What this means is that you’re purely testing the IOL’s optics here – assessing best distance acuity removes the possibility of variations in results that are caused by residual refractive error. I’d also suggest that you use a projector that randomizes the optotypes for the most accurate results.The next step is add additional negative lenses to blur the optotypes, and assess VA after the placement of each lens. Starting with a −0.5 D lens, and increase the defocus in −0.5 D increments until you reach −5.0 D, then repeat with positive lenses, starting at +0.5 D and increasing in +0.5 D increments until a defocus level of +2.5 D is reached. The results for your patient (or if you want to record your own data on the lens, an average of a group of patients) can be plotted, and the result is the defocus curve: an illustration of the IOL’s relationship between VA and distance. The defocus curve will have peaks and valleys – the peaks highlight zones of better vision, and valleys denote zones of poorer vision, providing a metric of the functional range of vision that is achieved with that particular IOL. Typically, diffractive bifocal IOLs exhibit their poorest performance at intermediate distance, whereas rotationally symmetric refractive multifocal IOLs show poorer performance at near distance (Figure 1). Diffractive echelette IOLs exhibit a sustained range of maximum VA (Snellen 20/20 or better) through a range of 1.5 D of defocus, and a full range of functional VA (Snellen 20/40 or better) over a range of 2.5 D of defocus. (Figure 2). In all cases, the defocus curve can be displayed along the x-axis with the introduction of lenses, allowing us to choose the type of addition, positive or even negative, to accommodate the patient’s individual needs.
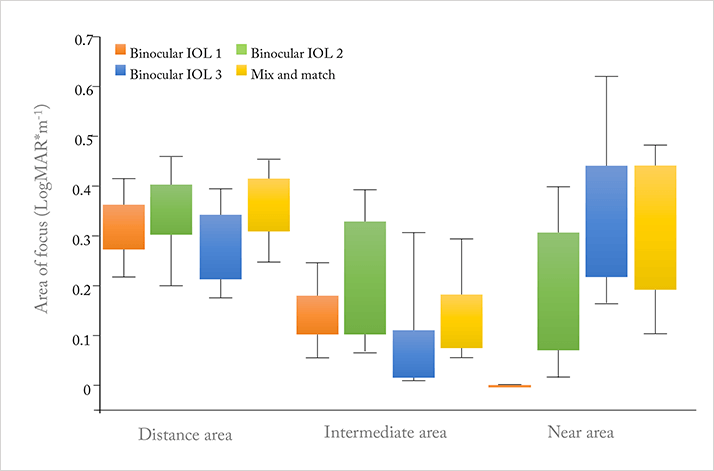
A modification of the conventional defocus curve based on area-of-focus metric has been proposed as a valid method of evaluation and differentiation between IOL designs (3) – area-of-focus provides an overview of the visual range separately for distance, near, and intermediate, (Figure 3) and could be a useful way of illustrating the differences between multifocal IOLs across these distances.
Measuring objective refraction
Ordinarily, retinoscopy and automatic refractometry are quick, easy and reliable techniques for measuring objective refraction – but the advent of the complex optical designs found in the latest IOLs means that the measurement of precise postoperative objective refraction has become more difficult – these techniques need to be used with caution (or with certain caveats) with some IOL designs under a number of circumstances. For example, we need to take into account that differing IOL materials will have a different Abbe number, meaning that they each have a different ability to disperse incoming light. As automatic refractometry works in infrared light, the estimated correction factor that is included in the system and was calculated for the crystalline lens will need to be changed depending on each IOL material. In general, retinoscopy has a slight tendency to provide more negative values in sphere and cylinder, generally below 0.5 D, after the implantation of diffractive multifocal IOLs (4), whereas retinoscopy tends to be more reliable with annular refractive-based IOL designs. However, compared with manifest refraction, automatic refractometry shows a strong tendency to produce more negative values – around 1 D in sphere and 0.5 D in cylinder (5). With zonal or segmented refractive-based designs, retinoscopy is only valid to verify IOL centration, as this method obtains direct and inverse retinoscopic reflexes simultaneously. Furthermore, automatic refractometry is not a reliable method for assessing cylinder with these IOLs. Why? It gives clinicians a mean value of measurements of all meridians, and those are obtained through both the distance and the near segments of the IOL. Additionally, the measured values should not be taken into account for the subjective refraction; instead, you should strongly consider assessing patients’ keratometric values as a starting point for refraction – these should almost certainly be unaffected by the type of IOL implanted.Subjective refraction
As modern IOL designs have multiple focal points, extended depth of focus or extended range of vision, I’d advise that, in order to avoid focus errors, you should start subjective refraction measurements from the hyperopic end. Further, I would say that it’s important to realize that classic tools for subjective refraction, such as the bichromatic test and JCCs also have to be used with caution in patients with these premium IOLs. Also, remember that if IOLs correct chromatic aberrations, then the red-green test won’t help. It still remains unknown how multifocality affects longitudinal chromatic aberration (LCA) – and as the bichromatic test is based on LCA, it really has to be performed under scotopic conditions if you want accurate, uncontroversial results. It’s a similar story with JCC – the blur induced during JCC may also be affected by multifocal IOLs, especially if the IOL is not well-centered. Indeed, if the induced coma-like aberration is over 0.1 µm, patient responses regarding the axis calculation may be unreliable and subject to misinterpretation, although the induction of spherical aberration does not affect the evaluation of the axis and cylinder power (6). If the multifocal IOL is well-centered but the kappa angle is high, it’s advisable to induce a dioptric change of 1 D or more through the JCC, in which case I believe that the best option is to use cross cylinders of ±0.5 D to guarantee the reliability of the test.Sphere adjustment is also important after multifocal IOL implantation. A range of plus and minus lenses are used to analyze the range of higher VA compared with the zero defocus. A starting point for the subjective refraction of the sphere is the middle point of this defocus interval. The important advantage of this technique is that it avoids the variability that could compromise a future refractive adjustment with laser. With monofocal IOLs the objective refraction usually is close to the subjective one, but still dependent on the Abbe number of the IOL material and its individual correction factor for infrared light. As now even the objective measurement has become more difficult to obtain, greater care has to be taken in evaluating a precise subjective refraction.
How to avoid focus error
The main source of error in refraction measurements after multifocal IOL implantation is focus error, as you’re performing the measurement for distance all the way through to the near focus of the IOL. Focus error is not common with diffractive IOLs, but it can happen with near-segment IOLs. If you suspect that focus error has occurred, here’s a three-step verification method you should consider employing:- Assess VA for distance, intermediate and near. If distance corrected near visual acuity is about 0.2 decimal (Snellen 20/100) then there is a high probability of focus error.
- Add a −2.50 D lens to the calculated distance refraction; if VA drops 2–3 lines, it’s likely that the refraction was correct.
- Add a +2.50 D lens to the calculated distance refraction; if VA drops to levels around 0.1 decimal (Snellen 20/200), then we can also consider that the refraction was correct.
However, if VA improves, your refraction is likely to be incorrect due to focus error (7).
Pathological conditions that may affect refraction
Certain pathological conditions may significantly mislead you when assessing patients’ refraction. One example is PCO, which can induce retinoscopic reflex attenuation – in fact, PCO with multifocal IOLs can induce a more dramatic reduction in VA than with monofocal IOLs (8). It’s important to remember that dry eye can induce transient astigmatisms too – treating your patients’ dry eye with lubricant drops or gels may help you to accurately assess the refractive status in such cases. Finally, during the immediate and recent postoperative period, it’s important to remember that one potential adverse event associated with cataract surgery and IOL implantation – cystoid macular edema (CME) – can provoke hyperopic shifts and reduce VA, so if CME is suspected, consider imaging the retina to find out.Conclusions
Implanting multifocal, extended depth of focus or extended range of vision IOLs makes refracting the patients that have received them a far more challenging and complex procedure than it is in patients with monofocal IOLs. Retinoscopy, keratometry and defocus curve assessments are the more reliable methods of performing this task, whereas autorefraction, the bichromatic test and JCC have significant limitations imposed not just by the optical characteristics of the IOLs, but also the presence of higher-order aberrations. Focus error, PCO, dry eye and CME are also sources of measurement errors and need to be accounted for. These IOLs are called “premium” for a reason – they can produce excellent outcomes and delight patients – but part of the reason why such IOLs have a price premium is that they involve considerably more work in planning and performing the surgery, and postoperative refraction assessment. Plan carefully, be aware of the caveats and work around them, and you should have patients that appreciate the premier service that goes into implanting a premium IOL.References
- K Hayashi, et al., “Effect of astigmatism on visual acuity in eyes with a diffractive multifocal intraocular lens”, J Cataract Refract Surg, 36, 1323–1329 (2010). PMID: 20656155 DP Piñero, et al., “LASIK outcomes following multifocal and monofocal intraocular lens implantation”, J Cataract Refract Surg, 26, 569–577 (2010). PMID: 19894668 PJ Buckhurst, et al., “Multifocal intraocular lens differentiation using defocus curves”, Invest Ophthalmol Vis Sci, 53, 3920–3926 (2012). PMID: 22589435 H Bissen-Miyajima, et al., “Autorefraction after implantation of diffractive multifocal intraocular lenses”, J Cataract Refract Surg, 36, 553–556 (2010). PMID: 20362844 G Muñoz, et al., “Validity of autorefraction after cataract surgery with multifocal ReZoom intraocular lens implantation”, J Cataract Refract Surg, 33, 1573–1578 (2007). PMID: 17720072 S Perches, et al., “Influencia de las aberraciones de alto orden en la determinación del astigmatismo mediante los CCJ”, e-poster. Optom2014 Meeting, Madrid (2014). C Albarrán-Diego, et al., “Prevention of hyperopic surprise after LASIK in patients with refractive multifocal intraocular lens”, Eur J Ophtalmol, 21, 826–829 (2011). PMID: 21667463 V Vrijman, et al., “Effect of Nd:YAG laser capsulotomy on refraction in multifocal apodized diffractive pseudophakia”, J Refract Surg, 28, 545–550 (2012). PMID: 22869233
