What led you into ophthalmology? I majored in chemistry and decided I would go to medical school. I thought I wanted to become a nephrologist. I traveled widely as a medical student: nephrology in San Diego, hematology at Tufts University, neurology in Guy’s Hospital London, and ophthalmology in Massachusetts Eye and Ear Infirmary. At that time, the ophthalmology department included laryngology and dermatology. Two months into my internship I realized it wasn’t for me. I felt like an over-glorified secretary and triage nurse. All I seemed to be doing was writing orders and other administrative work. I liked ophthalmology, I liked technology, and I thought I’d like optics. I decided that it would be more interesting.
Can you tell us about your work with targeting melanin with lasers?
I was at the point when my research grant was ready for renewal, I suggested we target melanin – as it is present throughout the trabecular meshwork. It just so happened that I was working in the department of dermatology at the time, and they were developing lasers for hair removal. It inspired me to consider whether lasers could target melanin within ocular structures; I thought that if we took a pulse laser and shortened the pulse duration to match it to the relaxation time of the melanin granule, we could target the melanin in the cell without affecting any surrounding cells or tissue structures.
How did you put your theory to the test?
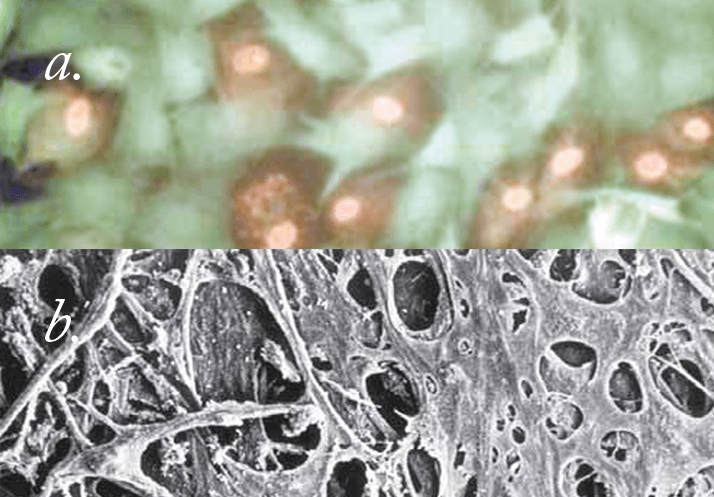
We used cell culture to begin with, just to check the technology and to see if we could do it. I created a system where we cultured pigmented trabecular meshwork cells that we incubated with melanin, and mixed them in with non-pigmented cells. We were able to demonstrate that with certain laser pulse durations – ideally with the 532 nm Q-switched laser – you could target the pigmented trabecular meshwork cell, whereas the non-pigmented trabecular meshwork cell right next to it would be totally unaffected. It worked well, and we published several papers based on
our studies.
So did you immediately seek medical applications? That went slowly! I went to several companies like Alcon and said, “Look I think I can develop this model that targets the trabecular meshwork and actually creates glaucoma”. They weren’t interested. It forced me to rethink my approach. I realized that maybe we don’t need to coagulate the trabecular meshwork to do trabeculoplasty and reduce the intraocular pressure (IOP). I thought that you might be able target those cells and get the same reduction of IOP by a creating a biological response in the meshwork. Companies would be much more interested if I had a glaucoma treatment, rather than a glaucoma model. So I turned the whole thrust of our research in the opposite direction.
At that stage did you go in vivo? After completing the in vitro cell culture studies, we decided to try this approach in monkeys, even though there was no good animal model for glaucoma. We were just about to publish our in-vitro results when I realized that it would be better to patent the technology before publishing! So you though that this cell targeting approach could be a treatment, but you didn’t really have any evidence? We were pretty sure we could target the cells, and we assumed that we didn’t need to photocoagulate anything: photocoagulation is like dropping an atomic bomb to kill a fly. I thought that if we could just target the cell and turn on the entire biological response system, we should get a similar pressure reduction without the destructive damage to the trabecular meshwork that photocoagulation produces. Our approach would also show that IOP lowering following laser is not a mechanical response, but a biological one.
How did you commercialize SLT? I presented our cell culture research to Jim Hobart, who was then the head of Coherent Medical Group. At the time, I had also developed a laser procedure to perform filtration surgery using a technique I invented called laser gonioscopic ab-interno laser sclerostomy. However, for various reasons the technique wasn’t successful, and the company that I had developed the laser with had little experience in ophthalmology. I therefore approached Coherent Laser (now Lumenis) which was the largest and most successful ophthalmic laser company in the world; Hobart agreed to fund the project and we set out to build a prototype laser. He understood the concept; he said yes let’s look into it and we’ll build you a laser suitable for in vivo monkey studies. If it was successful, the company would have rights of first refusal for licensing the technology.
What came next? We first demonstrated selective targeting in monkeys. I then tried to actually create glaucoma in the monkeys. Argon laser-induced scarring of the trabecular meshwork typically results in significant elevations in IOP – it’s called the Gasterland monkey model for glaucoma. But with our laser, we treated, and re-treated and re-treated. Whatever I did, I just couldn’t get the monkeys to develop glaucoma. We did the pathology and saw the meshwork looked quite normal; there was some depletion of pigmented cells, but overall it was healthy. That led us to believe it was relatively safe. So we had no choice, we had to build the laser to treat a patient. Was that done in the US? No, we took it to Mexico. The first patients we saw all had rather severe open angle glaucoma. I treated their trabecular meshwork in a manner somewhat similar to performing argon laser trabeculoplasty but using the laser parameters that I figured out based on our cell culture results.
What did you expect to see and what did you actually see in the first patient? We didn’t know what to expect; we had just no idea. But we lasered the patients’ eyes using low power and we found that the day after treatment, almost all the patients had a 50 percent reduction in pressure. We thought this was unbelievable. We had patients with pre-treatment pressures of 40 mmHg and they came back after treatment with pressures of 20 mmHg. These were people with bad eyes, some with end stage disease! Would you have dared hope for those results? We couldn’t have even imagined that result. So, at that point the project took off. I’ll never forget when we went to file for an FDA IDE (Investigational Device Exemption) and all the work and studies that followed.
There must have been an incredible amount of trepidation before you treated that first patient? Yes you’re right; we had no idea really how it would turn out. We could make the patient better or we could make the patient worse. Before treating the first patient, based on our monkey studies, I felt that the procedure was likely to be relatively safe. You knew it was safe but you didn’t know if it was going to be effective. Yes, if there had been a huge pressure elevation or the development of intractable glaucoma, the whole thing would have collapsed and that would have been the end of it.
So what was the reaction amongst your peers? People were interested, but our clinical trials were being conducted on eyes that were on maximum tolerated medical therapy, plus the protocol we set up was very conservative. Despite that, we were still getting good – 25 percent – pressure reductions in those patients. Did you come up against skepticism? I think there’ll always be skeptics. I’ll be honest with you, it’s taken 10 years because we would constantly hear “What’s the difference with what you are achieving, you’re still getting equivalent pressure reductions to argon laser trabeculoplasty?” The challenge was convincing them that there was no damage to the meshwork: you’ll be able to re-treat them because it’s a much gentler procedure, and more biologically specific than the argon laser, as you’re not getting its complications – you’re not producing peripheral anterior synechiae and you’re not scarring the trabecular meshwork. After a lot of development work with Lumenis, we were ready for the FDA… but they wouldn’t let us perform the trial with primary therapy. So we had to go back and do another multi-center clinical trial looking at selective laser trabeculoplasty (SLT) as primary therapy. Now that’s its main role Ophthalmologists have finally accepted that it’s safe, it’s effective – and maybe more effective than argon laser – plus, it doesn’t cause any damage to the outflow pathway, the trabecular meshwork. Patients do very well post-operatively, and there’s minimal inflammatory response.
Do you think the regulatory process is just a barrier? I think the FDA’s regulatory process plays an important role but they can also be a significant impediment to progress. The FDA’s role should be simple – show that a product or medication is relatively safe and has demonstrated efficacy for its intended purpose. Let the markets and physicians decide if the new therapy is going to be successful. There are just too many requirements and unnecessary concern about stuff that’s already been demonstrated. Duplication is a huge cost for companies and it’s basically holding back improvements in healthcare.
