
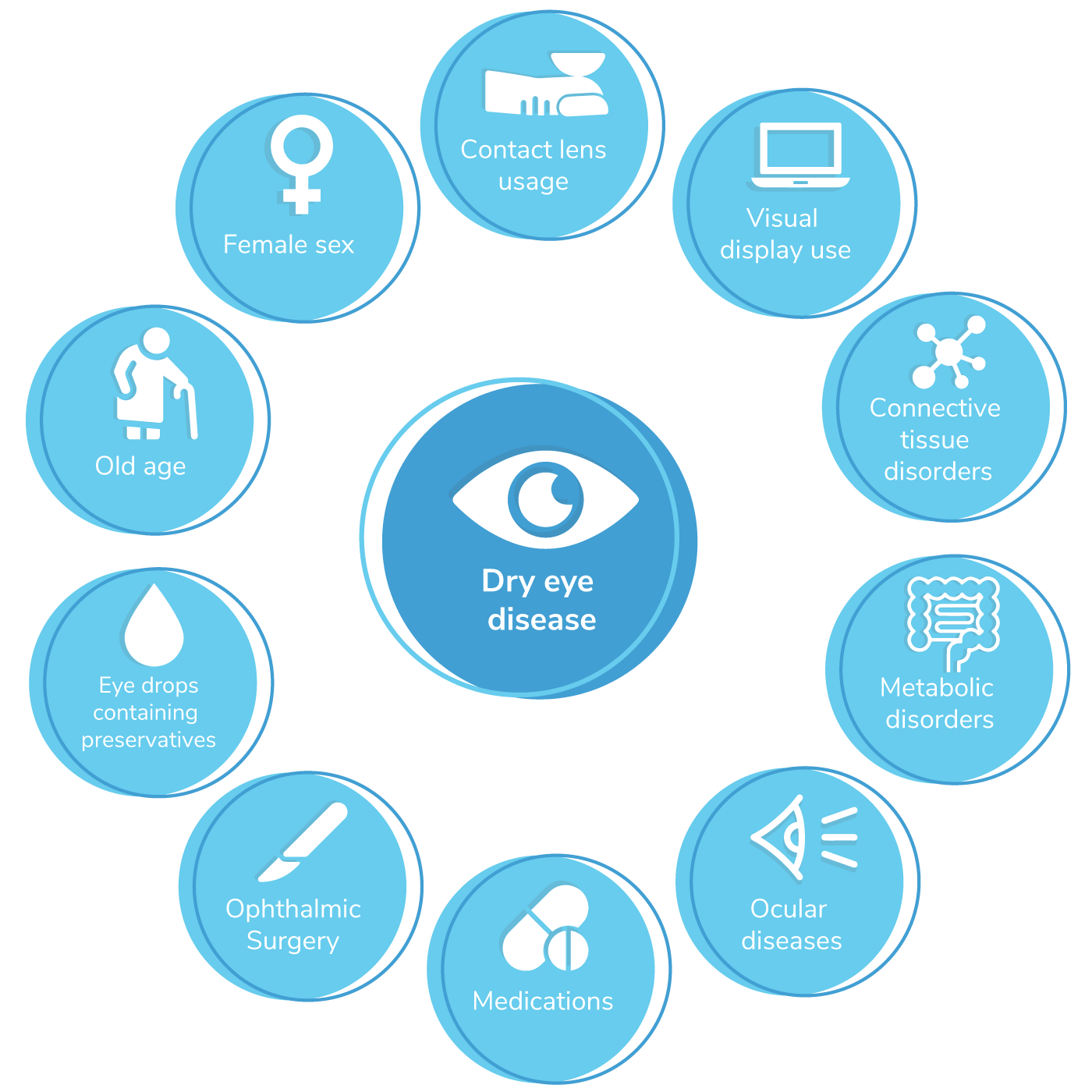
Dry eye disease (DED), also known as keratoconjunctivitis sicca, is reported in between 5 to 50 percent of adult patients globally, with women and the elderly being at a higher risk (1). The prevalence of DED in Asia – 27.1 percent – is reported to be higher than other continents, with prevalence in India, where we practice, varying between 18.4 and 54.3 percent (2, 3, 4, 5). According to a recent observational study, the average overall incidence of DED in India was 14,593/million population – or 1.58 percent in urban populations and 1.31 percent in people living in rural areas (6).
Individuals with DED experience ocular irritation, itching, redness, mucous discharge, foreign body sensation, photosensitivity, and visual blurring which have an impact on their daily activities (8). DED imposes a substantial negative impact on health-related quality of life and causes considerable economic burden due to loss of work productivity (9). Multiple risk factors cause or contribute to transient dry eye symptoms (see Figure 1).
Post-surgical DED
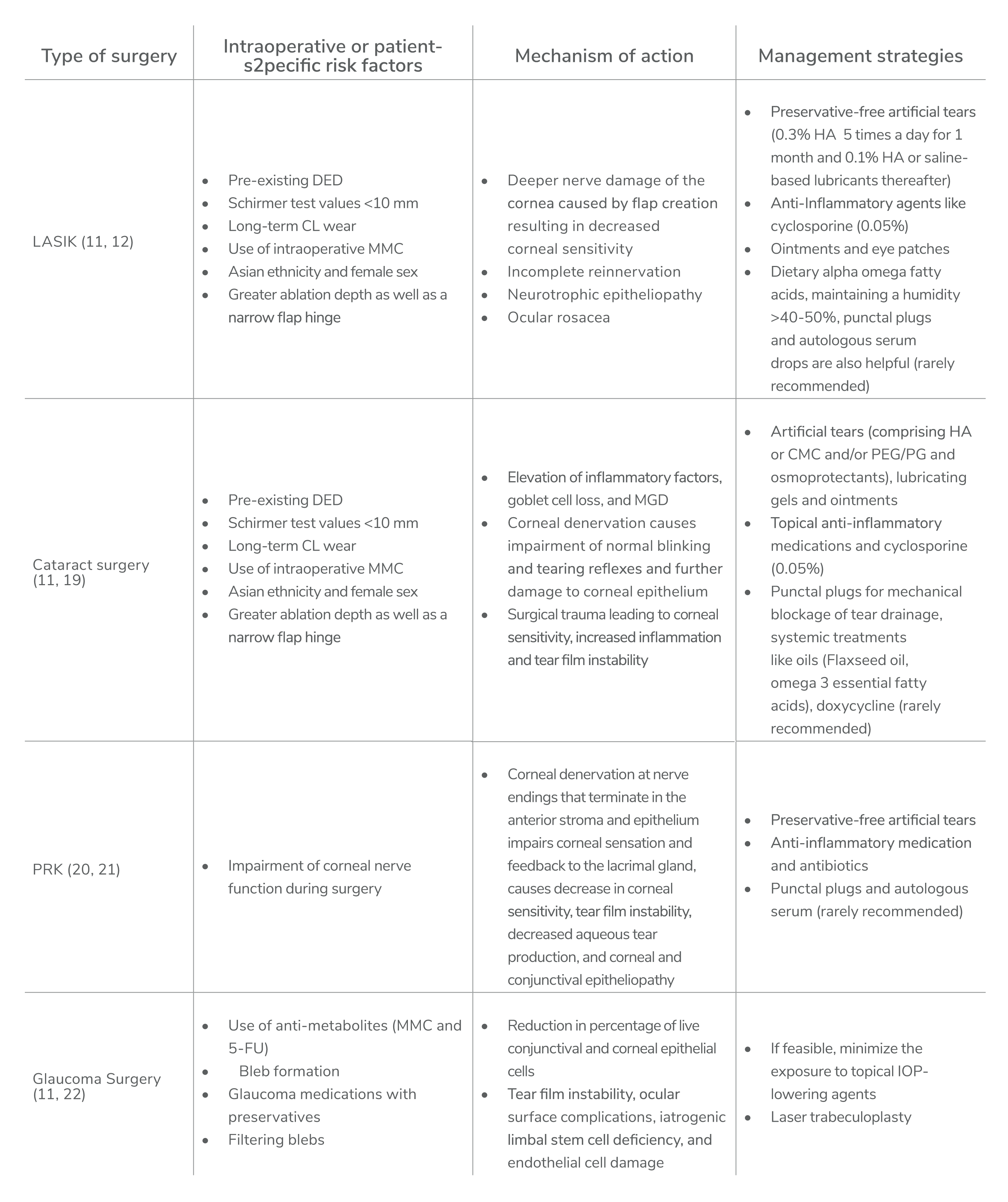
Surgeries to correct refractive errors, including LASIK, cataract surgery, PRK, SMILE, femtosecond lamellar extraction (FLEx), and other ophthalmic procedures (lid surgery, glaucoma surgery, keratoplasty, conjunctival surgery, vitreoretinal surgery) can all trigger dry eye symptoms transiently or exacerbate existing symptoms (11). LASIK is the most common procedure that affects tear film in approximately 50 percent of patients at 1 week, 40 percent at 1 month, 20–40 percent at 6 months, with 0.8 percent lasting more than one year (12, 13). The incidence and prevalence of DED after cataract surgery is 9.8 percent and 34 percent at four weeks and six months post-surgery, respectively – but the impact of surgery is difficult to gauge because pre-existing DED is frequent in patients with cataracts (14, 15).
Postoperative dry eye can have a negative impact on visual recovery outcomes and recovery time (16). Although the symptoms are transient in the majority of cases, it can affect the overall quality of life of the patient (11, 16). And patients may report dissatisfaction and postoperative discomfort because of dry eye symptoms (17, 18). Considering the ever-increasing number of patients undergoing the ophthalmic procedures highlighted above, it is important to understand the risk factors, mechanisms of action, and management strategies for each to reduce further complications and improve patient satisfaction and comfort (see Table 1).
General recommendations for other surgeries
Other ophthalmic procedures (lid surgery, glaucoma surgery, keratoplasty, conjunctival surgery, vitreoretinal surgery) can also result in dry eye symptoms. In general, pre-operative screening and management of post-surgical dry eye symptoms should be considered as critical aspects of pre- and postoperative planning. Aggressive topical lubrication, oral and topical anti-inflammatory agents, and punctal plugs can be recommended in susceptible eyes. Major intra-operative strategies include gentle manipulation, preservation of the conjunctival architecture post-peritomy, secure wound closure, minimized thermal cautery use, reduced surgical time to avoid prolonged corneal exposure, use of a corneal light shield, and frequent instillation of balanced salt solution (11).
Artificial tears in the management of post-surgical dry eye
Neurogenic inflammation caused by incision and ablation performed during refractive and cataract surgeries reduce corneal sensitivity; therefore, we need to use agents that promote healing via corneal re-epithelialization – either by increasing cells’ proliferation/migration rate, by aiding wound healing through modulating inflammation, or by facilitating cell attachment for wound closure (23). Previously published studies have reported that artificial tears can accelerate corneal re-epithelialization through different mechanisms (14, 24, 25). Artificial tears improve visual acuity, contrast sensitivity, corneal epithelial regularity, tear film stability, ocular surface stress, and also minimize desiccation and cell death by providing moisturization and lubrication to the ocular surface (26, 27, 28, 29, 30). Furthermore, peri- or post-surgical instillation of artificial tears has been shown to improve symptoms of dry eye, making them a crucial component for the management of post-surgical dry eye (11, 31). These buffered hypotonic or isotonic solutions contain viscosity-enhancing agents, osmotic agents, antioxidants, preservatives, and some inactive agents like buffers, electrolytes, and excipients (30).
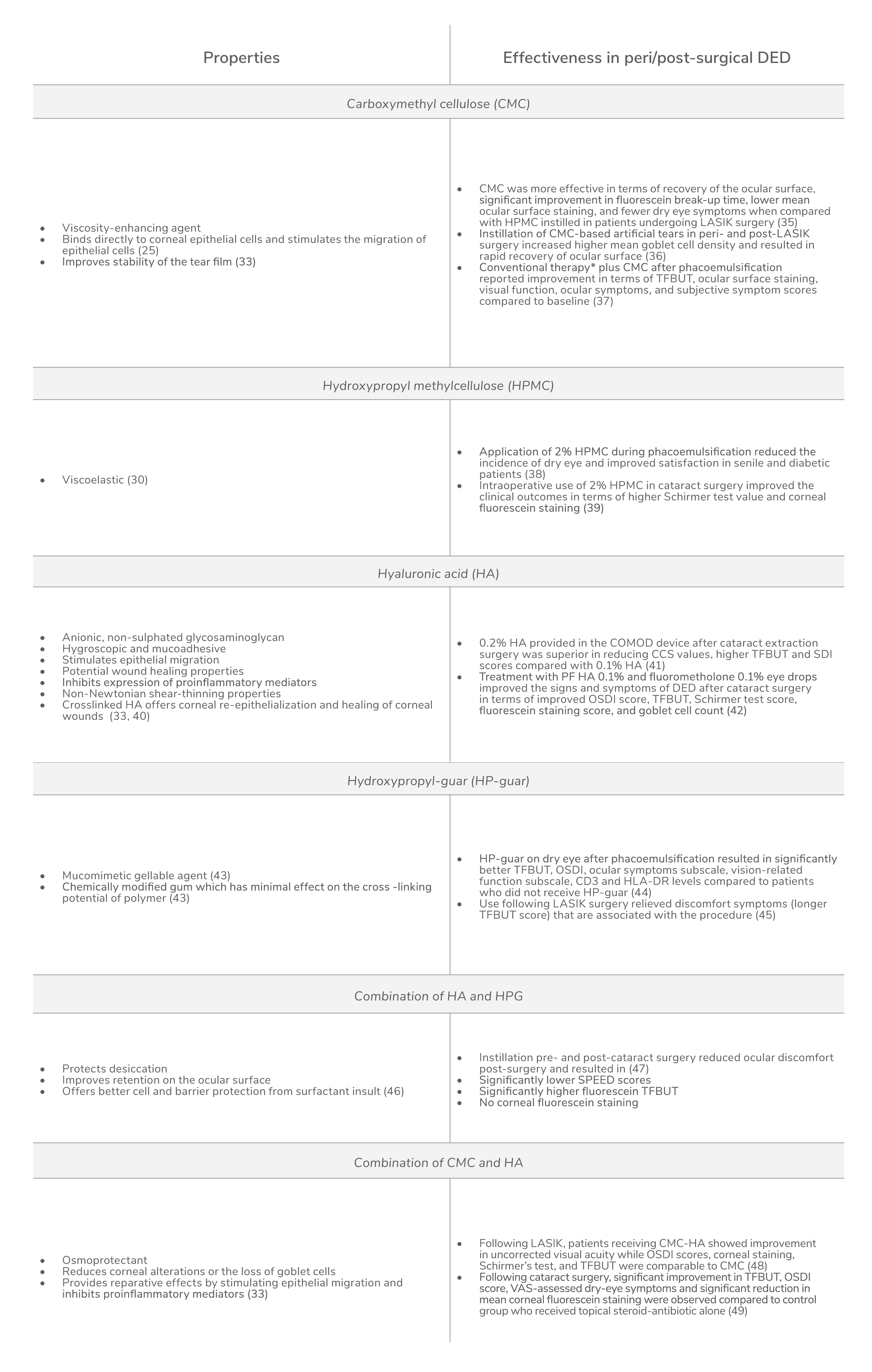
Next-generation tears
Artificial tears have now progressed to advanced gel-forming drops that contain natural or synthetic polymers create a more viscous and elastic matrix in the patient’s eye than saline products, resulting in increased duration of effect. Common viscosity agents include carboxymethyl cellulose (CMC), hyaluronic acid (HA), hydroxypropyl-guar (HP-guar), and hydroxypropyl cellulose. Acting alone or in combination (for example, HP-guar-HA), these viscosity enhancers increase tear film thickness, protect against desiccation, promote tear retention at the ocular surface, protect the ocular surface, maintain physiological corneal thickness, improve goblet cell density, and relieve dry eye symptoms (30). Each hydrogel formulation acts in a different way; for example, borate/HP-guar crosslinks with natural divalent ions in the tear film to form a soft gel (very low viscosity) that is maintained between the blinks (32). In other formulations, the synergic action of different polymers can contribute towards better lubrication and retention on the ocular surface than single-polymer based artificial tears – and, therefore, result in better symptom relief (33, 34). These viscosity-enhancing agents impart a number of clinical benefits in patients experiencing post-surgical dry eye symptoms (see Table 2).
Beyond viscosity
Different categories of ophthalmic demulcents, including cellulose derivatives, dextran, gelatin, and liquid polyols, may also be used in artificial tear formulations. And though high molecular weight polyhydric alcohol demulcents, such as PEG/PG, do not increase viscosity, they can mimic mucin layer and protect the corneal surface when in combination with gelling agents, such as HP-guar (50). Specifically, tear formulations based on PEG/PG and HP-guar combinations have been shown to provide relief from dry eye discomfort symptoms induced or exacerbated by LASIK or cataract surgeries (45, 51). In general, ophthalmic demulcents are considered vital components of prescription formulations for DED.
A final category of compounds, osmoprotectant, protect cells under extreme osmotic stress by balancing osmotic pressure without disturbing cell metabolism. Each osmoprotectant differs in kinetics of cell entry and exit, speed of action, and acts upon different processes; however, a fixed combination of osmoprotectants can have protective synergic effect against hyperosmolarity. The most commonly used osmoprotectants in artificial tears include L-carnitine, betaine, glycerol, erythritol, and trehalose (30, 33).
No time for tears?
Regardless of the type of surgery or the peri-operative and patient-specific risk factors at play, ocular surgeries quite often aggravate or result in dry eye symptoms. In the majority of cases, dry eye symptoms are transient in nature; here, artificial tears can be considered the first-line therapy. Indeed, studies have demonstrated that dry eye symptoms were improved – and, importantly, no safety concerns were raised – when artificial tears were instilled pre- or post-operatively. However, not all artificial tears are created equal! Those that promote corneal wound healing (via corneal re-epithelialization) and offer anti-inflammatory properties appear to be best placed to reduce the incidence of post-surgical dry eye symptoms. As to the future, we would like to see further systematic, randomized clinical trials to better understand the frequency and duration of instillation needed for consistent outcomes in patients undergoing refractive correction surgery.
References
- F Stapleton et al., “TFOS DEWS II Epidemiology Report,” Ocul Surf, 15, 334 (2017). PMID: 28736337.
- Y Wan et al., “The global prevalence of dry eye disease and its association with economy: a systematic review,” [Preprint] (2019).
- JS Titiyal et al., “Prevalence and risk factors of dry eye disease in North India: Ocular surface disease index-based cross-sectional hospital study,” Indian J Ophthalmol, 66, 207 (2018). PMID: 29380759.
- N Gupta et al., “Estimating the prevalence of dry eye among Indian patients attending a tertiary ophthalmology clinic,” Ann Trop Med Parasitol, 104, 247 (2010). PMID: 20507698.
- S Shah, H Jani, “Prevalence and associated factors of dry eye: Our experience in patients above 40 years of age at a Tertiary Care Center,” Oman J Ophthalmol, 8, 151 (2015). PMID: 26903719.
- PR Donthineni et al., “Incidence, demographics, types and risk factors of dry eye disease in India: Electronic medical records driven big data analytics report I,” Ocul Surf, 17, 250 (2019). PMID: 30802671.
- JD Nelson et al., “TFOS DEWS II Introduction,” Ocul Surf, 15, 269 (2017). PMID: 28736334.
- EM Messmer, “The pathophysiology, diagnosis, and treatment of dry eye disease,” Dtsch Arztebl Int, 112, 71 (2015). PMID: 25686388.
- M McDonald et al., “Economic and Humanistic Burden of Dry Eye Disease in Europe, North America, and Asia: A Systematic Literature Review,” Ocul Surf, 14, 144 (2016). PMID: 26733111.
- ME Stern et al., “Dry eye as a mucosal autoimmune disease,” Int Rev Immunol, 32, 19 (2013). PMID: 23360156.
- JAP Gomes et al., “TFOS DEWS II iatrogenic report,” Ocul Surf, 15, 511 (2017). PMID: 28736341.
- I Toda, “Dry eye after LASIK,” Invest Ophthalmol Vis Sci, 59, DES109 (2018). PMID: 304881814.
- A Nemet et al., “Post laser-assisted in-situ keratomileusis dry eye disease and temporary punctal plugs,” Indian J Ophthalmol, 68, 2960 (2020). PMID: 33229678.
- R Mecucci et al., “Iatrogenic Dry Eye Disease: Dealing with the Conundrum of Post-Cataract Discomfort. A P.I.C.A.S.S.O. Board Narrative Review,” Ophthalmol Ther, 10, 211 (2021). PMID: 33555571.
- K Naderi et al., “Cataract surgery and dry eye disease: A review,” Eur J Ophthalmol, 30, 840 (2020). PMID: 32515220.
- M Dalton, Ophthalmology Times, “Managing dry eye key to patient satisfaction after cataract, refractive surgeries” (2019). Available at: https://bit.ly/2YXqlHA.
- P Garg et al., “Dry eye disease after cataract surgery: Study of its determinants and risk factors,” Turkish J Ophthalmol, 50, 133 (2020). PMID: 32630999.
- G Rabina et al., “The Association between Preoperative Dry Eye Symptoms and Photorefractive Keratectomy,” J Ophthalmol (2019). PMID: 31275633.
- U Devgan, Review of Ophthalmology, “Dry-eye syndrome after cataract surgery” (2005). Available at: https://bit.ly/3aHF9MQ.
- GG Quinto et al., “Postrefractive surgery dry eye,” Curr Opin Ophthalmol, 19, 335 (2008). PMID: 18545018.
- KS Bower et al., “Chronic dry eye in PRK and LASIK: manifestations, incidence and predictive factors,” J Cataract Refract Surg, 41, 2624 (2015). PMID: 26796443.
- H Ji et al., “Dry eye disease in patients with functioning filtering blebs after trabeculectomy,” PLoS One, 11, e0152696 (2016). PMID: 27032098.
- BD Ashby et al., “Corneal injuries and wound healing – review of processes and therapies,” Austin J Clin Ophthalmol, 1, 1017 (2014).
- Y Zhang et al., “Evaluation of artificial tears on cornea epithelium healing,” Int J Ophthalmol, 11, 1096 (2018). PMID: 30046523.
- Q Garrett et al., “Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing,” Investig Ophthalmol Vis Sci, 48, 1559 (2007). PMID: 17389485.
- MR Nilforoushan et al., “Effect of artificial tears on visual acuity,” Am J Ophthalmol, 140, 830 (2005). PMID: 16310460.
- Z Liu, SC Pflugfelder, “Corneal surface regularity and the effect of artificial tears in aqueous tear deficiency,” Ophthalmology, 106, 939 (1999). PMID: 10328393.
- S Cohen et al., “Evaluation of clinical outcomes in patients with dry eye disease using lubricant eye drops containing polyethylene glycol or carboxymethylcellulose,” Clin Ophthalmol, 8, 157 (2014). PMID: 24403819.
- JH Lee et al., “Efficacy of sodium hyaluronate and carboxymethylcellulose in treating mild to moderate dry eye disease,” Cornea, 30, 175 (2011). PMID: 21045674.
- L Jones et al., “TFOS DEWS II Management and Therapy Report,” Ocul Surf, 15, 575 (2017). PMID: 28736343.
- E Cohen et al., “Dry eye post-laser-assisted in situ keratomileusis: Major review and latest updates,” J Ophthalmol (2018). PMID: 29619255.
- U Benelli, “Systane lubricant eye drops in the management of ocular dryness,” Clin Ophthalmol, 5, 783 (2011). PMID: 21750611.
- AJM Orobia et al., “Combination of hyaluronic acid, carmellose, and osmoprotectants for the treatment of dry eye disease,” Clin Ophthalmol, 12, 453 (2018). PMID: 29563769.
- M Labetoulle et al., “Efficacy and safety of dual-polymer hydroxypropyl guar-and hyaluronic acid-containing lubricant eyedrops for the management of dry-eye disease: A randomized double-masked clinical study,” Clin Ophthalmol, 12, 2499 (2018). PMID: 30584269.
- JM Albietz et al., “A comparison of the effect of refresh plus and bion tears on dry eye symptoms and ocular surface health in myopic LASIK patients,” CLAO J, 28, 96 (2002). PMID: 12054380.
- LM Lenton, JM Albietz, “Effect of carmellose-based artificial tears on the ocular surface in eyes after laser in situ keratomileusis,” J Refract Surg, 15, S227 (1999). PMID: 10202728.
- K Yao et al., “Efficacy of 1% carboxymethylcellulose sodium for treating dry eye after phacoemulsification: Results from a multicenter, open-label, randomized, controlled study,” BMC Ophthalmol, 15, 28 (2015). PMID: 25880685.
- M Yusufu et al., “Hydroxypropyl methylcellulose 2% for dry eye prevention during phacoemulsification in senile and diabetic patients,” Int Ophthalmol, 38, 1261 (2018). PMID: 28699061.
- Y He et al., “The improvement of dry eye after cataract surgery by intraoperative using ophthalmic viscosurgical devices on the surface of cornea: The results of a consort-compliant randomized controlled trial,” Med (Baltimore), 96, e8940 (2017). PMID: 29390284.
- C Posarelli et al., “Cross-linked hyaluronic acid as tear film substitute,” J Ocul Pharmacol Ther, 35, 381 (2019). PMID: 31373862.
- P Ntonti et al., “Impact of 0.1% sodium hyaluronate and 0.2% sodium hyaluronate artificial tears on postoperative discomfort following cataract extraction surgery: a comparative study,” Eye Vis, 6 (2019). PMID: 30805405.
- D Jee et al., “Comparison of treatment with preservative-free versus preserved sodium hyaluronate 0.1% and fluorometholone 0.1% eyedrops after cataract surgery in patients with preexisting dry-eye syndrome,” J Cataract Refract Surg, 41, 756 (2014). PMID: 25487027.
- I Petricek et al., “Hydroxypropyl-guar gellable lubricant eye drops for dry eye treatment,” Expert Opin Pharmacother, 9, 1431 (2008). PMID: 18473717.
- MA Sánchez et al., “The effect of preservative-free HP-Guar on dry eye after phacoemulsification: A flow cytometric study,” Eye, 24, 1331 (2010). PMID: 20300126.
- D Durrie, J Stahl, “A randomized clinical evaluation of the safety of Systane® Lubricant Eye Drops for the relief of dry eye symptoms following LASIK refractive surgery,” Clin Ophthalmol, 2, 973 (2008). PMID: 19668456.
- R Rangarajan et al., “Effects of a hyaluronic acid/hydroxypropyl guar artificial tear solution on protection, recovery, and lubricity in models of corneal epithelium,” J Ocul Pharmacol Ther, 31, 491 (2015). PMID: 26067908.
- E Favuzza et al., “Protecting the ocular surface in cataract surgery: The efficacy of the perioperative use of a hydroxypropyl guar and hyaluronic acid ophthalmic solution,” Clin Ophthalmol, 14, 1769 (2020). PMID: 32616996.
- A Wallerstein et al., “Management of post-LASIK dry eye: A multicenter randomized comparison of a new multi-ingredient artificial tear to carboxymethylcellulose,” Clin Ophthalmol, 12, 839 (2018). PMID: 29765198.
- R Mencucci et al, “Effect of a hyaluronic acid and carboxymethylcellulose ophthalmic solution on ocular comfort and tear-film instability after cataract surgery,” J Cataract Refract Surg, 41, 1699 (2015). PMID: 26432128.
- MB Abelson, R Anderson, Review of Ophthalmology, “Demistifying Dumulcents” (2006). Available at: https://bit.ly/3mZ9EDG.
- G Labiris et al., “Impact of polyethylene glycol 400/propylene glycol/hydroxypropyl-guar and 0.1% sodium hyaluronate on postoperative discomfort following cataract extraction surgery: a comparative study,” Eye Vis (Lond), 4, 13 (2017). PMID: 2849070.
