
As some of you may know, the original maker of the Raindrop corneal inlay, Revision Optics (RVO), abruptly closed its doors on January 30, 2018. On October 22, 2018, the US Food and Drug Administration issued an FDA Safety Communication – a recall of the Raindrop corneal inlay (1). This FDA recall followed the FDA’s premarket approval of the Raindrop corneal inlay on June 29, 2016 (2).
I was an FDA investigator in the original clinical trial. The best estimate of the number of Raindrop corneal inlays implanted during the period of commercial implantation is between 2,000 and 3,000. The initial reason for the FDA recall was the development of corneal haze that could affect clear vision – but this did not address the other, more significant issues associated with untreated corneal haze, including impending corneal melt, corneal melt, and corneal melt with extrusion of inlay.
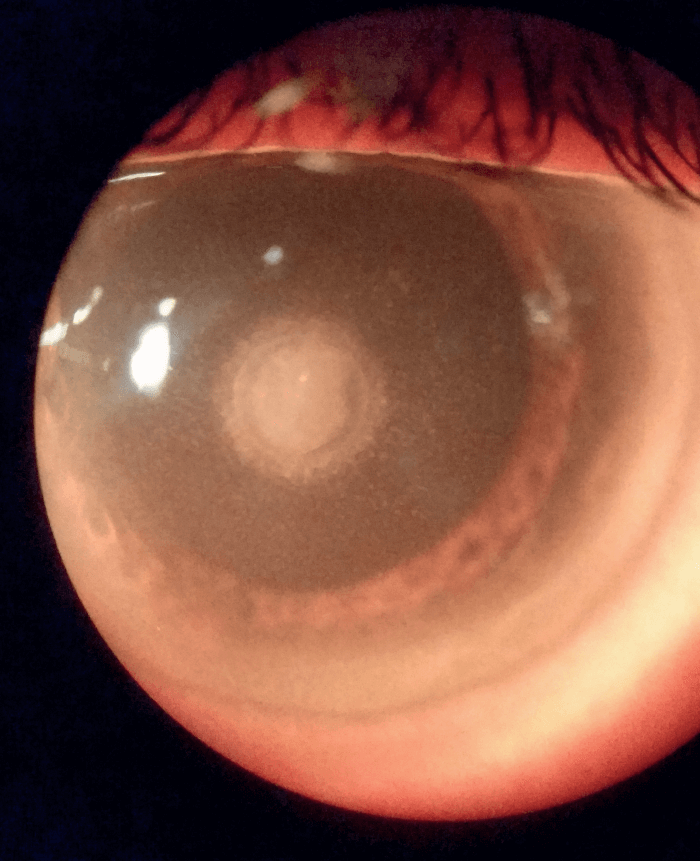
I have now removed all of the inlays I implanted as part of the FDA trial, but I have recently seen a cluster of patients with the known late complications of the Raindrop corneal inlay. These patients were implanted in other settings; they are now presenting with severe haze and impending corneal melt.
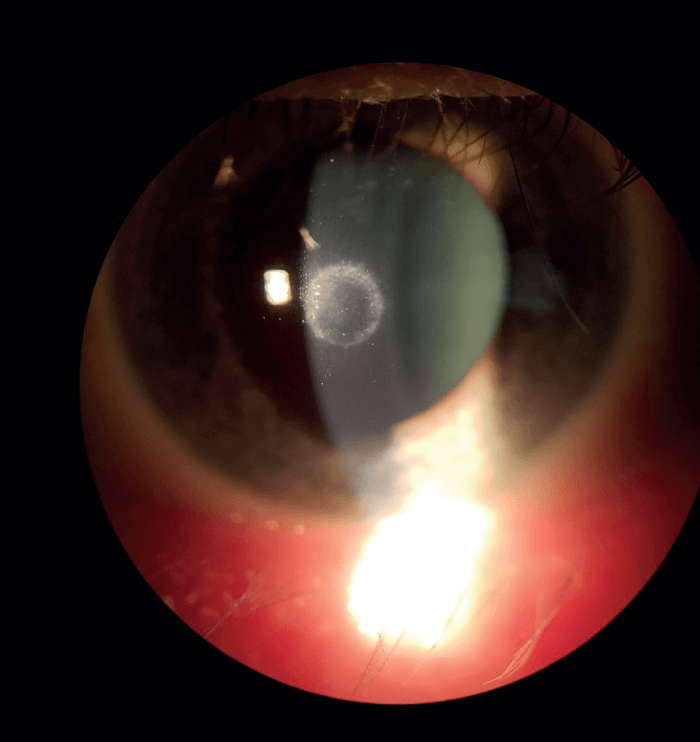
Since October 2018, efforts have been made to contact every patient implanted with the Raindrop to notify them of the recall and offer them appropriate treatment options, ranging from inlay explantation to more frequent follow-up examinations. However, some patients have clearly not responded to the notifications and others have chosen to continue with the inlay despite the known risk. There was clearly a corneal haze complication “honeymoon” period noted in the FDA trial; this was extended during commercial implantation by burying the inlay deeper in the cornea and using mitomycin C to delay the development of corneal haze. The original honeymoon period in the FDA trial lasted for about a year and a half after implantation, so the cluster of cases I have just seen coincides with what was shown in the trial.
I strongly suggest that every surgeon who implanted a Raindrop corneal inlay should contact every remaining inlay patient and advise them of the need for continuing follow-up appointments until the inlay is explanted. It should also be noted that there are known cases of haze-related problems occurring six months after explantation of the device. Although we do not know the magnitude of the problem, we do know that there are still Raindrop corneal inlays in eyes out there – and that they are potential ticking timebombs.
References
- US FDA, “Increased risk of corneal haze associated with the raindrop near vision inlay: FDA safety communication” (2018). Available at: https://bit.ly/3hyxmBG.
- US FDA, “Premarket Approval (PMA)” (2016). Available at: https://bit.ly/3hxPEDl.
