
Early in 2019, I interviewed Robert MacLaren, renowned vitreoretinal surgeon, Professor of Ophthalmology at the University of Oxford, UK, for the cover feature of our March 2019 issue, while coming back from an event in London. The signal on my train journey was really bad, and I remember hanging out of the window to make sure I could hear him well. It was worth the effort, as it was one of the most interesting and inspiring conversations I've ever had. Here is what MacLaren told me.
Gene therapies are no longer just hype – they provide tangible benefits to real patients, today. What disease will next fall to this powerful modality? For a clue, look at the biggest gene therapy trial in the world.
Gene therapy has been hyped for many years, in many therapy areas – but for now, it may be ocular disease that benefits most. Regulators have already approved a gene therapy for Leber Congenital Amaurosis (LCA), and similar products are currently being developed for several other diseases of the eye. Some of these are now in clinical trials and attracting commercial interest. Overall, the field is moving very quickly, and it’s an exciting time to be involved, not least in my own field: gene therapies for choroideremia and X-linked retinitis pigmentosa.
REP-1 repair
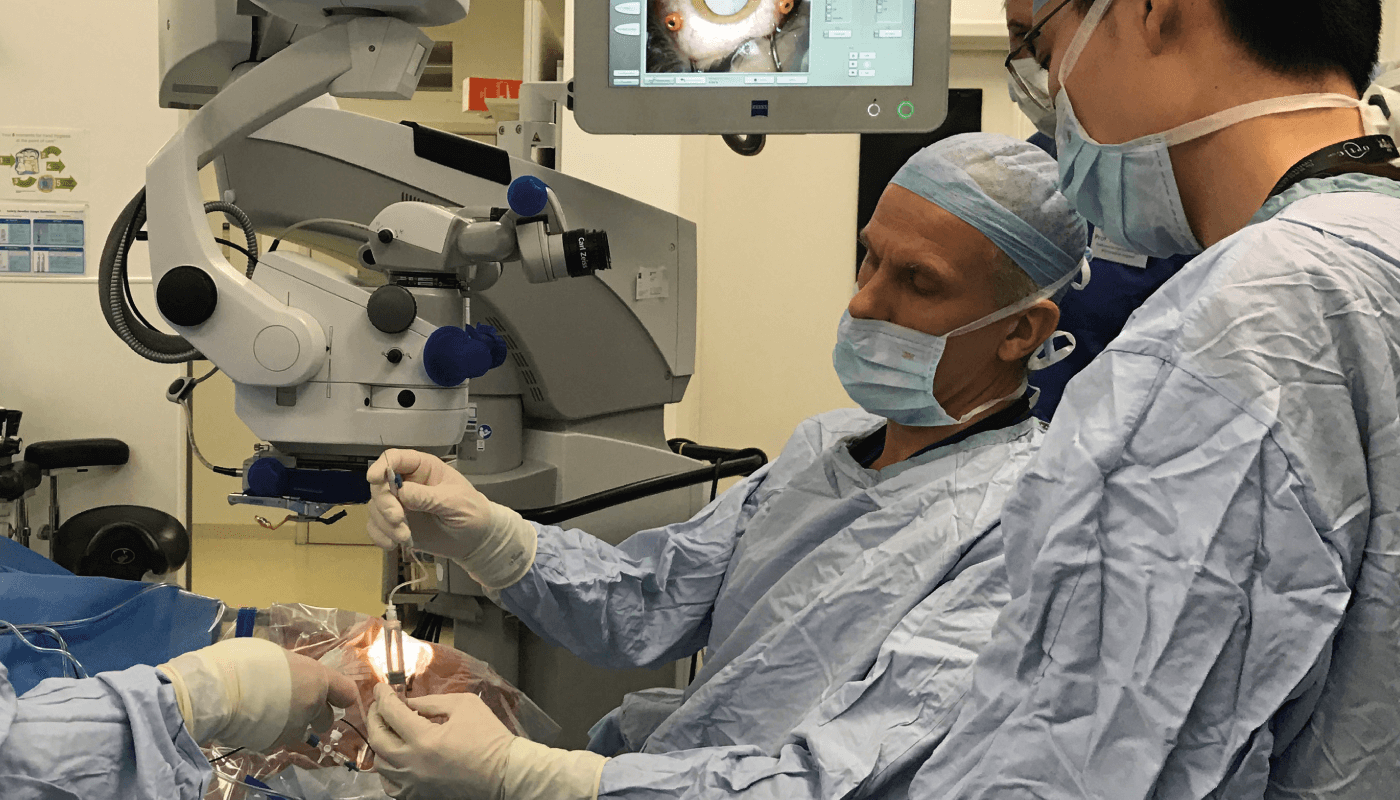
Choroideremia is an X-linked retinopathy caused by deficiency in the REP-1 protein. The condition progresses to blindness, and there is no treatment. The need for a new therapeutic option is therefore acute. I have been working on a choroideremia gene therapy for several years; if successful, it will represent the first treatment for this problematic disease. Our approach involves the construction of a small retinal detachment so as to form a subretinal space, followed by injection of AAV2-vectored REP-1 sequences into this space. The method has generated very encouraging data from Phase I and Phase II trials: in brief, at the two-year follow-up of a 14-patient trial, treated eyes exhibited a median visual acuity gain of 4.5 Early Treatment Diabetic Retinopathy Study chart letters, while untreated fellow eyes showed a median decline of 1.5 letters (1).
These promising data enabled our choroideremia treatment to progress to the commercial stage – it is being taken forward by Nightstar Therapeutics, an Oxford University spin-out funded primarily by the Wellcome Trust. Nightstar has now initiated a clinical trial involving study sites spread across 11 countries. It’s the biggest gene therapy trial anywhere – and we’re very excited about it.
One down…
After Nightstar took on our choroideremia gene therapy, we set our sights on another target – X-linked retinitis pigmentosa (XLRP). This disorder, which primarily affects males, is associated with photoreceptor loss caused by mutations in the RPGR gene. Again, the condition leads to blindness and is at present incurable. We intend to change that.
This project, however, was complicated by inherent features of the RPGR gene. RPGR contains a purine-rich region, with highly repetitive nucleotide sequences, which is genetically unstable. In consequence, the cloning steps necessary to insert RPGR into a viral vector are associated with unpredictable recombination errors in this region. We solved this problem by developing a codon-optimized RPGR sequence which, when expressed from an AAV8 backbone, provides RPGR protein equivalent to wild-type protein. Thus, our XLRP gene therapy comprises an AAV8 vector carrying codon-optimized RPGR DNA.
That work cleared the way for the world’s first XLRP treatment, trialed here in Oxford in March 2017. Since then, our XLRP gene therapy has progressed to larger-scale trials in various countries including the UK and the US. The forward momentum has been assisted by the clinical network we built up during our choroideremia program – the same surgeons that want to cure choroideremia also want to deal with XLRP. Again, this project is now being commercialized by Nightstar Therapeutics.
Reasons to be cheerful (Part 3 and beyond)
We have other programs in our pipeline, including AMD gene therapy. Looking further ahead, some aspects of glaucoma may have a genetic predisposition, suggesting a role for gene therapy in this condition too. And although most of our efforts at present are directed at low-hanging fruit in the gene therapy tree – which is to say, augmentation of non-functional genes – I think the future will see us go after more difficult targets. These include dominant diseases where we must not only provide a therapeutic sequence but also remove or disable the original dysfunctional sequence. The CRISPR gene-editing system has obvious applications in this approach.
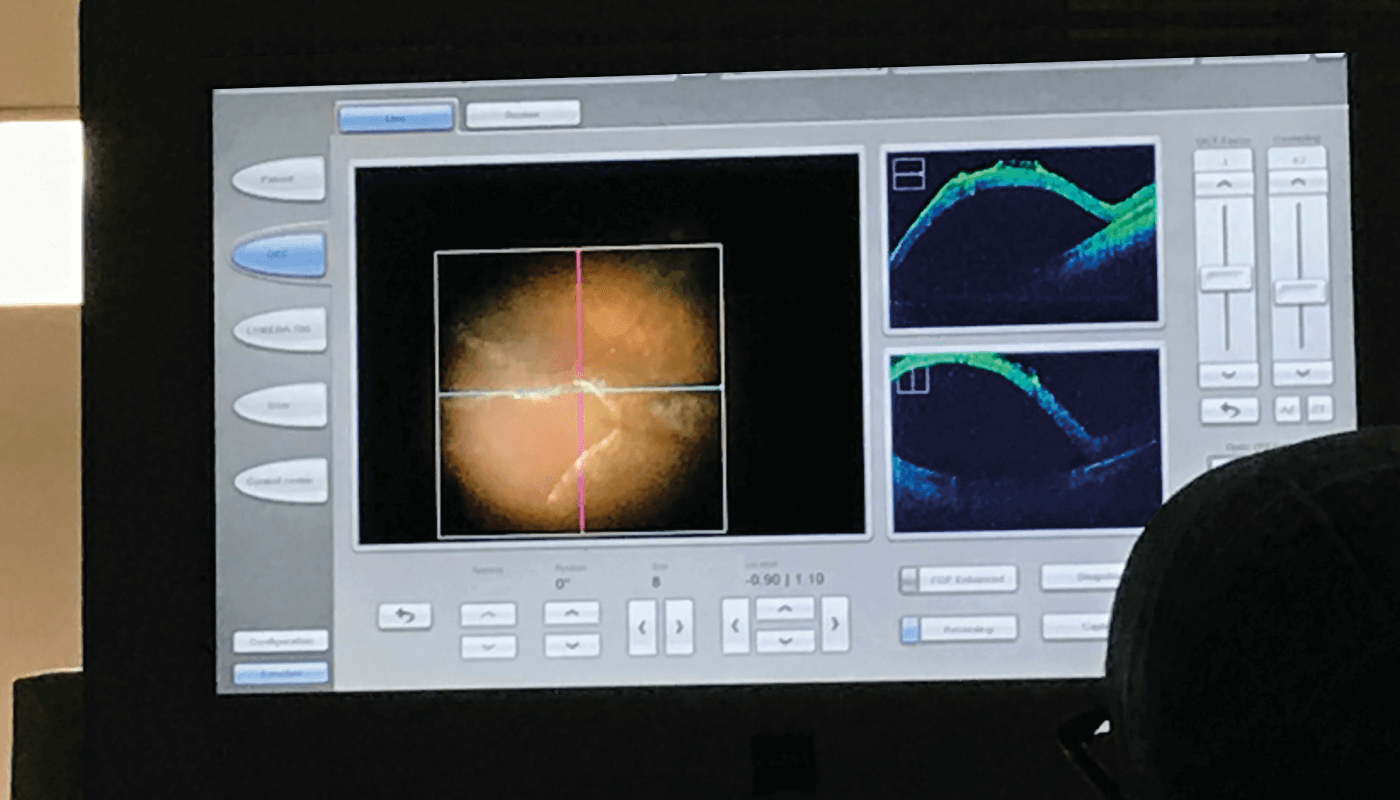
In all cases, however, we should remember that an essential part of retinal gene therapy is delivery of therapy to the retina! At present, we rely on delicate surgical expertise - but to realize the full potential of retinal gene therapy, we’ll probably need to develop new surgical techniques. Recent developments include intra-operative OCT – which, for example, allows us to carefully monitor the iatrogenic retinal detachment step in our choroideremia gene therapy – and robot-mediated ocular surgery. But whatever developments tomorrow brings, it is clear that exciting days lie ahead for ocular gene therapy.
Robert MacLaren is a consultant to Nightstar Therapeutics, Spark Therapeutics and Gyroscope Therapeutics. He sits on the UK Department of Health evaluation committee, which assesses new retinal gene therapy treatments.
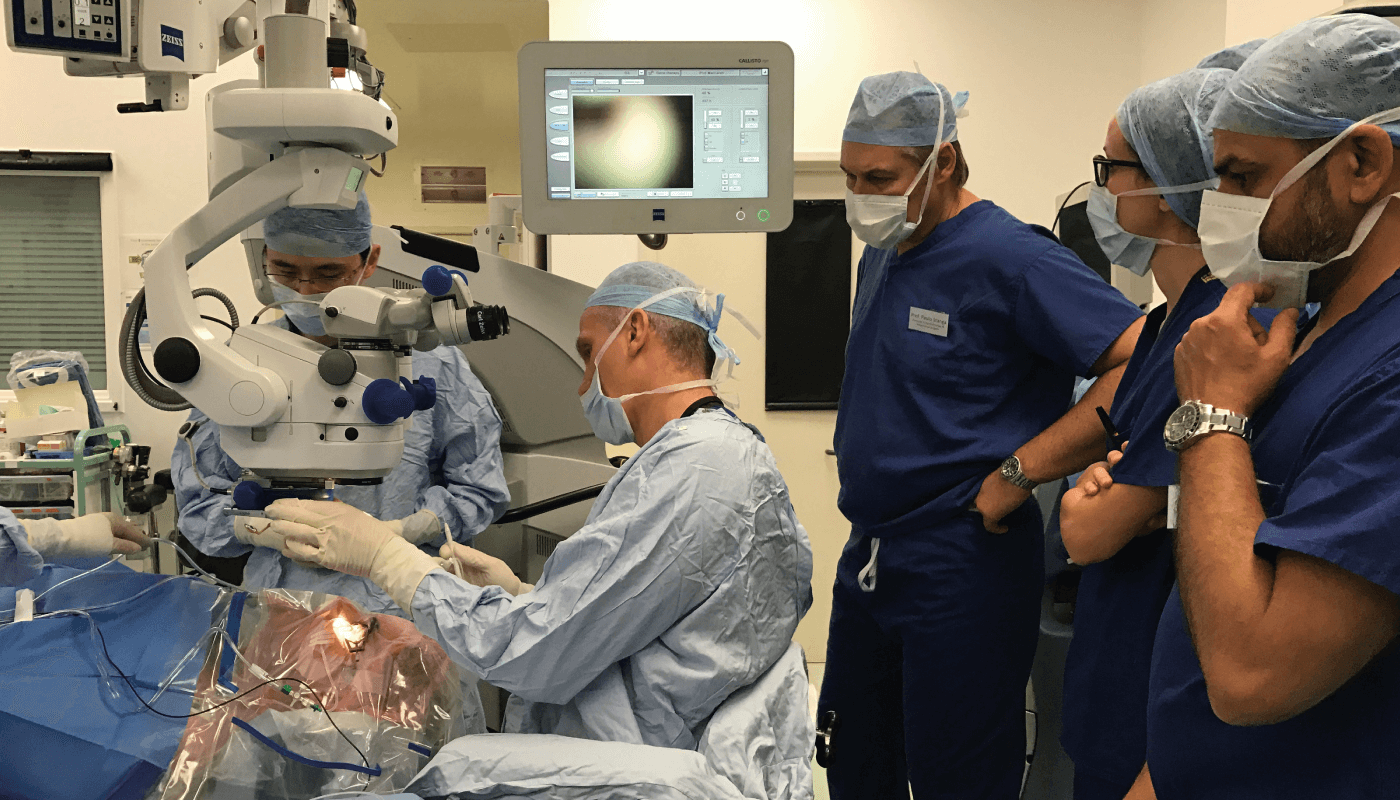
A few months later, once again I had the opportunity to interview MacLaren, this time focusing on his inspirations, career highlights, and his advice for younger colleagues. Read the Sitting Down With... to find out what he told me.
