David A. Goldman, MD - Founder of Goldman Eye, Palm Beach Gardens, Florida, and Ophthalmology Team Lead, Anterior Segment, Modernizing Medicine, USA, talks about his experience with Upneeq® (oxymetazoline hydrochloride ophthalmic solution), 0.1% from RVL Pharmaceuticals – an FDA-approved treatment for acquired ptosis in adults.
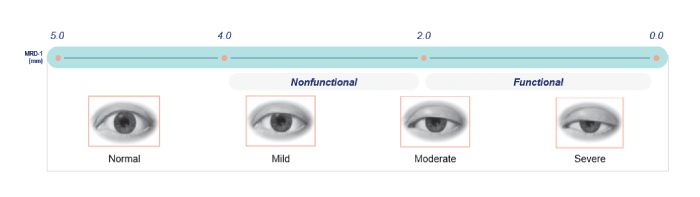
My practice consists of two ophthalmologists and one optometrist. We take care of everything covered by comprehensive ophthalmology, including the various forms of ptosis. Acquired ptosis in adults is prevalent in my practice – especially in the elderly patient demographic, which is the majority of our patient population. The condition’s prevalence is, however, much better separated into those who medically require surgery and those who seek surgery for cosmetic benefit. There is wide variation in ptosis severity, from minimal to severe (requiring surgery) – but even patients with mild ptosis will often ask about their lid surgery eligibility.
Widening the patient pool
The introduction of Upneeq has completely changed how I treat acquired ptosis across the board. Before, medically indicated ptosis patients would be referred to an oculoplastic specialist for surgery; elective patients with less severe presentation would also be referred, but without the likelihood of insurance coverage for the procedure. Now that Upneeq is available, I can offer treatment to patients with milder acquired ptosis, enabling patients with lesser functional infringements and cosmetic impact to address their condition. And the increased reach doesn’t just relate to acquired ptosis severity – one of Upneeq’s advantages is that the subset of acquired ptosis patients in whom surgical repair is contraindicated (such as those on blood thinners) may now have the opportunity to improve their field of vision. For some patients, field of vision improvements can potentially lead to noticeable impacts on day-to-day activities such as driving, reading, and sports. However, it’s important to keep in mind that Upneeq may not be right for all patients. Since Upneeq may impact blood pressure, physicians should tell patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension or hypotension to monitor their condition and call the office if it worsens.
The benefits extend to mild ptosis, in which patients may experience significant self-consciousness over asymmetry between lids or wish to make their eyes look more open. Acquired ptosis patients can now treat the appearance of their eyelids before any social or professional event without the need for surgery. This is especially valuable in mild ptosis patients who aren’t quite ready for surgery yet; operating too early can increase the risk of worse ptosis outcomes in older age or scarring following the procedure.
I see patients who have had lid surgery performed by a plastic surgeon (rather than an oculoplastic surgeon) who has removed the skin, but did not address the levator muscle. This will naturally droop over time and either be difficult to rectify with surgery or leave insufficient skin for a successful outcome. The non-surgical therapeutic option offered by Upneeq is appropriate for such patients – and it may be helpful for them.
Changes in practice As I mentioned, in the past, patients interested in lid surgery were referred to an oculoplastic specialist. Now, the nonsurgical option of prescribing Upneeq provides a stepping stone for apprehensive patients. Many of my patients have been so happy with the results from the eye drop that they asked to continue with a prescription in place of surgery.
There has been a significant trend in which our patients who have started using Upneeq are very satisfied with their clinical outcomes. By giving samples to patients and taking before and after pictures (15 minutes apart), my colleagues and I can quickly show patients the visible benefits – we even let appropriate patients test Upneeq on the spot in the office. Our staff educates patients on how to correctly instill eye drops and reminds patients not to touch the tip of the container to their eye or any other surface to avoid contamination. We also discuss potential adverse events which may occur in 1-5 percent of patients, including punctate keratitis, conjunctival hyperemia, dry eye, blurred vision, instillation site pain, eye irritation and headache. Patients are very curious about the product and ask us – “How long has this been available?” and “Why haven’t I heard of this before?” I have received comments such as, “I’ve got to tell my friends about this.” Their excitement and willingness to talk to friends about their satisfying experiences is fantastic advertising – perfect for generating further interest from other patients who may have been ignoring their acquired ptosis.
Unexpected ptosis diagnosis
A common complaint following cataract surgery is that patients feel their eyelids have become droopy. This is often not the case, but patients frequently go from wearing corrective glasses that can cover acquired ptosis to having a clear view of their eyelids. For this reason, we take a picture before cataract surgery so that patients can see for themselves whether there has been a change. Situations like this are often perfect for the prescription of Upneeq®, because the patient’s potential disappointment at that point can be addressed with a noninvasive prescription that avoids another surgery. This is also an example in which quick and early diagnosis of ptosis is extremely useful – diagnosing a patient before surgery is considered a pre-existing condition, whereas diagnosis afterwards can be considered a complication in the patient’s mind. It’s also important to keep in mind that ptosis may be associated with neurologic or orbital diseases and consideration should be given to these conditions during diagnosis. By managing the patient’s expectations and flagging any eye health concerns early, cataract surgery is much more likely to have a great outcome for everyone involved.
Flow and distribution
My practice staff talk to patients about ptosis before I’ve even seen them. Having the staff give a brief and informal introduction to the condition and the options available is brilliant both for the patients and for me – leading to many appropriate patients keen to try the prescription instead of surgery. We even have one staff member who has become an Upneeq user and is delighted with the effect it has on her unilateral acquired ptosis.
Upneeq’s distribution model makes the process easy for me and my patients. Because it works outside of insurance, it’s been easy to go through RVL for the prescription. The pricing of the product is appropriate for what it delivers; it won’t be affordable for everyone but, given the results that many of my patients see, it’s definitely a fair price for the service it performs.
My advice to any ophthalmologists who may want to start using Upneeq in their practice is to give their acquired ptosis patients samples and offer to write them a prescription. This, combined with taking before and after pictures with patients’ own cell phones, is typically a good way to ensure a successful launch of Upneeq in any ophthalmic practice.
IMPORTANT SAFETY INFORMATION
INDICATION UPNEEQ® (oxymetazoline hydrochloride ophthalmic solution), 0.1% is indicated for the treatment of acquired blepharoptosis in adults.
WARNINGS AND PRECAUTIONS
• Ptosis may be associated with neurologic or orbital diseases such as stroke and/or cerebral aneurysm, Horner syndrome, myasthenia gravis, external ophthalmoplegia, orbital infection and orbital masses. Consideration should be given to these conditions in the presence of ptosis with decreased levator muscle function and/or other neurologic signs.
• Alpha-adrenergic agonists as a class may impact blood pressure. Advise UPNEEQ patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension or hypotension to seek medical care if their condition worsens.
• Use UPNEEQ with caution in patients with cerebral or coronary insufficiency or Sjögren’s syndrome. Advise patients to seek medical care if signs and symptoms of potentiation of vascular insufficiency develop.
• UPNEEQ may increase the risk of angle closure glaucoma in patients with untreated narrow-angle glaucoma. Advise patients to seek immediate medical care if signs and symptoms of acute narrow angle glaucoma develop.
• Patients should not touch the tip of the single patient-use container to their eye or to any surface, in order to avoid eye injury or contamination of the solution.
ADVERSE REACTIONS
Adverse reactions that occurred in 1-5% of subjects treated with UPNEEQ were punctate keratitis, conjunctival hyperemia, dry eye, blurred vision, instillation site pain, eye irritation and headache.
DRUG INTERACTIONS
• Alpha-adrenergic agonists, as a class, may impact blood pressure. Caution in using drugs such as betablockers, anti-hypertensives, and/or cardiac glycosides is advised. Caution should also be exercised in patients receiving alpha adrenergic receptor antagonists such as in the treatment of cardiovascular disease, or benign prostatic hypertrophy.
• Caution is advised in patients taking monoamine oxidase inhibitors which can affect the metabolism and uptake of circulating amines.
Dr. David Goldman is a paid consultant of RVL Pharmaceuticals, Inc.
©2021 RVL Pharmaceuticals, Inc. All rights reserved.
Upneeq® is a registered trademark of RVL Pharmaceuticals, Inc.
References
- J Bacharach et al., Eye (Lond) [Online ahead of print] (2021). PMID: 33927356.
- Y Wang et al., Graefes Arch Clin Exp Ophthalmol, 257, 394 (2018). PMID: 30203103..
