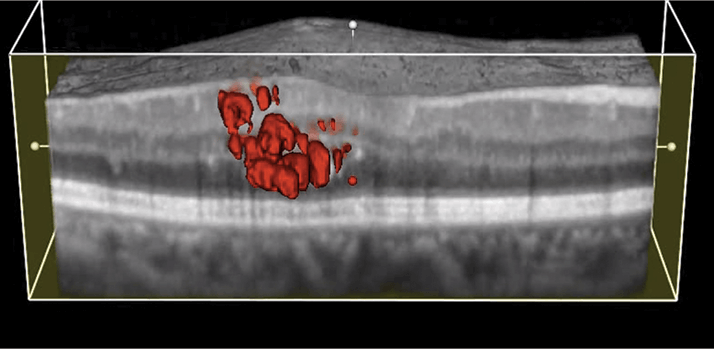
While it is the undisputed gold standard for wet AMD treatment today, treatment outcomes with intravitreal administration of anti-VEGF agents vary from patient to patient. Many comorbidities have been identified as contributing to this variation (1). A new addition to that list is the configuration of the vitreomacular interface (VMI); three different reports have concluded that VMI disease (VMID) can have a clinically important effect on visual outcomes and need for retreatment.
Last year, Amy Green-Simms and colleagues at the Mayo Clinic in Rochester, MN, USA, performed a retrospective case series analysis of patients with AMD who received intravitreal anti-VEGF injections and who either did (n=32) or did not (n=146) have VMID (2). They found that eyes with VMID had similar best corrected visual acuity (BCVA) to VMID-free eyes, but that they required more anti-VEGF therapy to achieve it. Those findings were confirmed by Ulrike Mayer-Sponer and colleagues at the Vienna Reading Center in Vienna, Austria, who performed a subanalysis of data from a phase III clinical trial that enrolled treatment-naïve patients with subfoveal choroidal neovascularization (3). The patients were randomized to receive ranibizumab therapy either every month or every three months. The team found that patients with VMID (in this case vitreomacular adhesion and release of vitreomacular contact) had to have the intensive, monthly treatment regimen to gain the full benefit of ranibizumab; patients receiving quarterly dosing experienced significantly poorer visual outcomes.
In March 2014, during the 66th Annual Wills Eye Conference, Samuel K. Houston III presented data from a retrospective consecutive case series that included patients with (n=51) and without VMA (n=153) (4). He reported that the groups had similar visual acuity (which improved over the two-year study period) and similar central retinal thicknesses. Yet, he noted that more intensive treatment was necessary for patients with VMA. “The longest interval extension occurred in the non-VMA group and was statistically significant,” Houston said, “The anatomic factors may contribute to individual treatment responses and should be evaluated and considered in treatment decisions for neovascular AMD.” Comorbidities like VMID have distinct effects on how regularly patients must be treated with ranibizumab. Few patients behave like the “average patient” of clinical trials, arguing that tailored anti-VEGF treatment regimens for AMD is required.
References
- S. Waldstein, “Predicting Anti-VEGF Treatment Outcomes”, The Ophthalmologist, 3, 26–28 (2013). A.E. Green-Simms et al., “Visual and anatomical outcomes of anti-vascular endothelial growth factor therapy in exudative age-related macular degeneration and vitreomacular interface disease: vitreomacular adhesion and epiretinal membrane” , Retina, 33, 1359–1364 (2013). doi:10.1097/IAE.0b013e3182845d18. U. Mayr-Sponer et al., “Influence of the Vitreomacular Interface on Outcomes of Ranibizumab Therapy in Neovascular Age-related Macular Degeneration”, Ophthalmology, 120, 2620–2629 (2013) doi: 10.1016/j.ophtha.2013.05.032. S.K. Houston, “Vitreomacular interface affects anti-VEGF injection intervals for AMD”. Oral presentation at the 66th Annual Wills Eye Annual Conference, Philadelphia, Pennsylvania, March 6–8th (2014).
