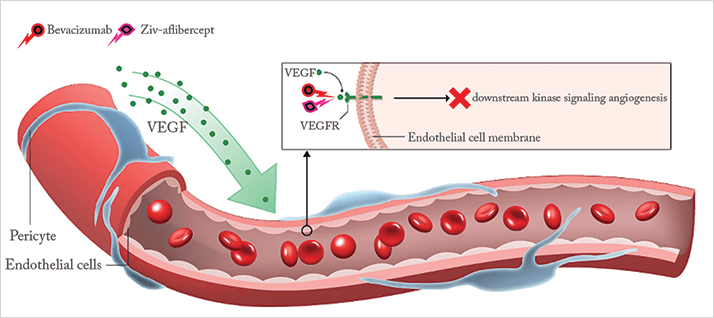
Have you ever wondered why there are two international nonproprietary names (INNs) for one very well-known VEGF inhibitor? Regeneron designed and produced the recombinant fusion protein, aflibercept; they (with Bayer) went on to develop it for ophthalmic use (INN: aflibercept [Eylea]); and partnered with sanofi-aventis to develop it for use (in combination with other chemotherapeutic agents) in treating metastatic colorectal cancer. In this latter context, its INN is ziv-aflibercept (Zaltrap). The FDA’s reasoning was that INNs should vary if they have: “different marketing applications held by different manufacturers, different formulations [particularly osmolality, at ~250 mOsm vs. ~ 820 mOsm], different routes of administration [intravitreal vs. intravenous]; and are [each] manufactured at different sites” (1). Despite these differences, thrifty ophthalmologists have identified a potential way of saving money: use ziv-aflibercept in the eye.
The osmolarity differences could be a dealbreaker – hyperosmolar solutions injected intravitreally can result in retinal toxicity and even retinal detachment (2), but a study of ziv-aflibercept in rabbit eyes and human retinal cultured cells (ARPE-19) found no evidence of any toxic effect (3). The next (and very brave) step would be to test ziv-aflibercept in human eyes. One research team based at the American University of Beirut in Lebanon did exactly that (4): six consecutive patients (four with AMD, two with DME) received intravitreal injections of 0.05 mL ziv-aflibercept – meaning that they received just a 1.25 mg dose – if the patients had received regular, ophthalmic aflibercept the dose administered would have been 2 mg – but 1.25 mg is still within the concentration range where aflibercept has shown therapeutic efficacy in patients with AMD (5).
Despite the lower dose, all six patients experienced an increase in visual acuity after one week. Patients’ mean logMAR visual acuity improved from 1.40 at baseline to 0.86 at one week post-injection; mean central macular thickness had decreased from 482 µm at baseline to 345 μm after seven days. But ultimately this exercise was about the cost-savings associated with Zaltrap instead of Eylea – which the study authors estimated to be about 20 times lower – potentially making ziv-aflibercept cheaper than bevacizumab for ophthalmic use. But back to reality – this was six patients, receiving one injection, with a one-week follow-up period. Administering intravitreal injections of ziv-aflibercept will continue to rest in the realms of the brave until considerably more data is available on its use. The study authors concluded: “It could also provide a second line of therapy in eyes with wet AMD or DME resistant to bevacizumab therapy in underprivileged countries”.
References
- Center for Drug Evaluation and Research, “Application Number: 125418orig1s000. Proprietary Name Review(s)”. Available at: http://bit.ly/zivaflibercept. Accessed August 27, 2015. MF Marmor, “Retinal detachment from hyperosmotic intravitreal injection”, Invest Ophthalmol Vis Sci, 18, 1237–1244 (1979). PMID: 116971. JR de Oliveira Dias, et al., “Preclinical investigations of intravitreal ziv-aflibercept”, Ophthalmic Surg Lasers Imaging Retina, 45, 577–584 (2014). PMID: 25423640. AM Mansour, et al., “Ziv-aflibercept in macular disease”, Br J Ophthalmol, 99, 1055–1059 (2015). PMID: 25677668. U Schmidt-Erfurth, et al., “Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies”, Ophthalmology, 121, 193–201 (2014). PMID: 24084500.
