
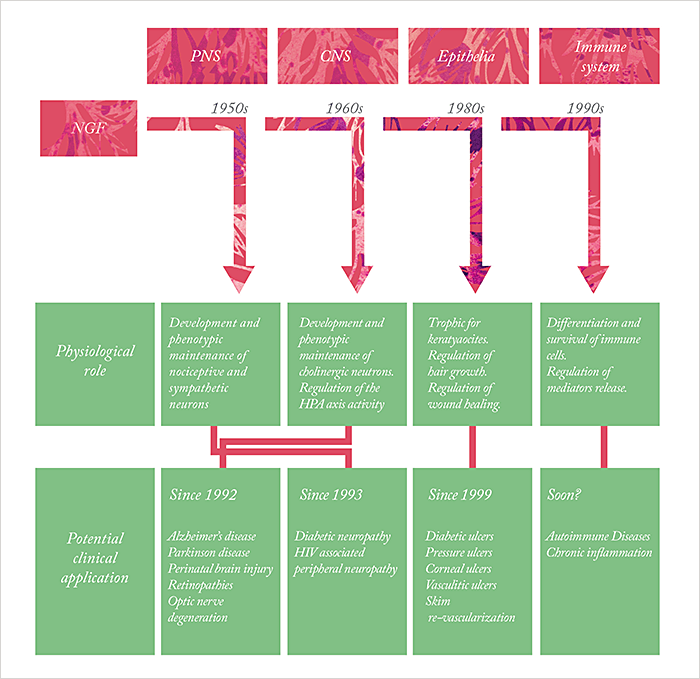
Rita Levi-Montalcini led an interesting life. Born in Turin on April 22nd 1909, she wanted to become a writer in her teenage years. Instead, she went to the University of Turin’s medical school, graduating with an MD in 1936, and worked in the laboratory of the noted neurobiologist, Giuseppe Levi. Political events conspired to take that position away from Levi-Montalcini – Hitler’s growing influence over Benito Mussolini meant that in 1938, Il Duce introduced the Manifesto of Race, which banned Jews from positions in government, banking and education, robbing Rita of her job. But Rita continued her work – in her bedroom in her Turin home, examining the growth of nerve fibers in chicken embryos, then after the Germans invaded Italy in 1943, from a corner of the shared living space in a building in Florence, where she and her family had fled. In 1947, Rita took a position in Viktor Hamburger’s laboratory at St. Louis’ Washington University, and it was there that she and the biochemist Stanley Cohen made the discovery that would, over three decades later, win them the 1986 Nobel Prize in Physiology or Medicine. Levi-Montalcini had observed that when tumors from mice were transplanted to chick embryos, they induced potent growth of the chick embryo nervous system – specifically sensory and sympathetic nerves, even when there was no direct contact between chick embryo and tumor. Rita’s conclusion was simple: the tumor releases a “nerve growth-promoting factor” that had a selective action on certain types of nerves. Stanley Cohen’s contribution was to use his skills as a biochemist to help the Hamburger lab identify and purify what was then called simply “nerve growth factor”, or NGF – and he went on to discover a very useful, enriched source of NGF, the mouse salivary gland, and later, to uncover another trophic factor: epidermal growth factor (EGF).
Since then, NGF’s role in the developing (and mature) body has been widely characterized, as has its part in a wide array of disease states – not just the growth, maintenance and survival of neurons. It also appears to have therapeutic applications in conditions ranging from acromegaly to Wegener’s granulomatosis, and from melanoma to… myopia correction. And it doesn’t stop there. It seems that this small-but-mighty molecule could have a number of therapeutic applications, both in the anterior and posterior segment – and if only a small portion of the theories become reality, NGF could turn out to be a game-changer for ophthalmology.
Rita Levi-Montalcini Facts:
- First ever Nobel laurate to reach 100 years of age
- The tenth woman to be elected to the US National Academy of Sciences
- One of three Nobel laureates who were tutored by Giuseppe Levi
- Founder and first president of the European Brain Research Institute
- Despite being a professed atheist, became a member of the Pontifical Academy of Sciences in 1974
- Made a Senatore a vita (Senator-for-life) in the Italian Senate in 2001

NGF and the anterior segment
If we start with the front of the eye, topical NGF therapy is currently being investigated for the treatment of three disorders: ocular surface disease, ocular surface/ corneal sensation issues after laser refractive surgery (PRK and LASIK), and neurotropic keratitis (NK). Neurotropic keratitis To better understand NGF’s role in NK it’s important to understand how NK develops. When corneal sensory nerves are damaged, the cornea suffers. It’s an avascular tissue, so the cornea relies heavily on its innervation for trophic support. When those nerves are damaged, it compromises the ability of the cornea to heal, and kickstarts a vicious circle: patients start to lose reflex tear secretion, leading to reduced protection of the eye. But if there is damage to the eye, patients aren’t going to feel it, notice, or seek treatment. So a lesion of the corneal epithelium can easily progress to corneal melting, and then on to corneal perforation. As NGF acts to stimulate not just nerve proliferation, but also epithelial cell proliferation, this means that in theory, it can tackle both pathologies. Paolo Rama, Chief of the Cornea and Ocular Surface Unit, at the Ospedale San Raffaele di Milano-IRCCS, Milan, tells of how NGF was first used to treat a patient with NK.It was back in Venice in 1996, and we had one patient in our clinic who had only one eye. She had presented with a painless white corneal infiltrate that was caused by a Candida infection – we couldn’t understand how she got infected with Candida in a healthy eye! After the resolution of the infection a deep corneal ulcer remained and on inspection, we discovered that she had a congenital aplasia of the trigeminal nerve that had resulted in corneal anesthesia, and as she was unaware of the pain, she had developed a very deep neurotrophic ulcer. Together with Alessandro Lambiase we decided to try NGF under compassionate use. We received murine NGF from Levi-Montalcini’s lab, and we treated her topically with it. After 10 days, the ulcer was completely healed, and after 6 months, the girl could see 10/10 through that eye – perfect vision. And so we started treating some other neurotrophic ulcers, and again, we saw that it worked. We presented the data to the New England Journal of Medicine, and it was accepted. Everything started from there (1).
The study Rama describes involved 12 patients (and 14 eyes), and topical murine NGF (mNGF) use resulted in a rapid healing of all of the ulcers, improved corneal sensitivity in most of them and improved visual acuity (1). Similar results were seen in a later study where mNGF was used to treat NK that was non-responsive to conventional treatments (2). Complete healing of the corneal defect was achieved in all patients between 12 days and 6 weeks after mNGF treatment was initiated. Side effects were also described: hyperemia and moderate pain in the eye and periocular area, but these were well tolerated and were expressed only during the period where mNGF treatment was necessary for corneal keratitis remission. A later study of 11 patients with NK (3) showed similar efficacy (complete ulcer healing in 9–43 days) and investigated mNGF’s adverse event profile in more detail, revealing that ocular discomfort was associated with eyedrop instillation, but this lasted for less than one hour and such painful sensations disappeared after the corneal ulcers healed – even when NGF continued to be applied. One concern with NGF use is the possibility that patients’ immune systems would begin to recognize mNGF as non-self, but this study found no trace of anti-mNGF antibodies during the period in which therapy was administered, nor during the follow-up period of up to 72 months. Today, recombinant human NGF (rhNGF) is available (see Box: Making rhNGF), and a multinational, multicenter Phase I/II clinical trial of topical rhNGF eye drops for the treatment of NK has recently been completed (NCT01756456), and a similar trial in the US is underway (NCT02227147).
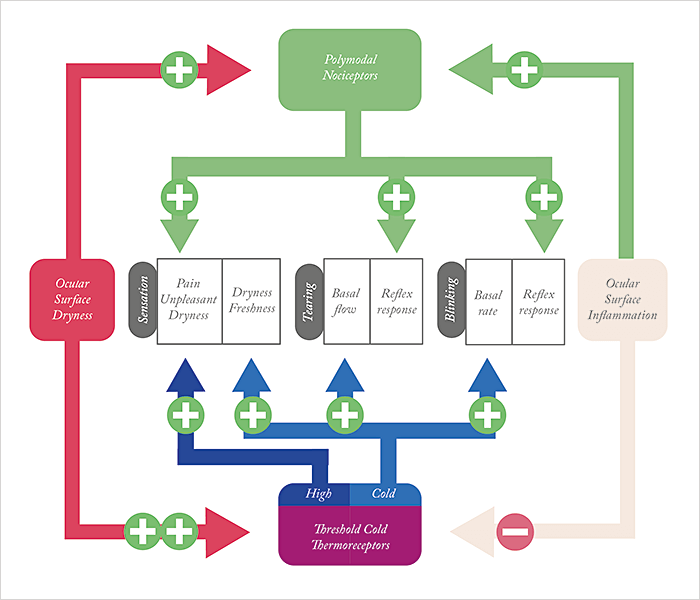
Dry eye disease The etiology of dry eye disease (DED) is massively multifactorial, but it’s clear that corneal nerve dysfunction plays a role – and an increasingly important one as the disease progresses (4). There are four main types of sensory receptors in the cornea – low threshold mechanoreceptors, high threshold mechanoreceptors, polymodal nociceptors, and cold thermoreceptors (CTRs). The mechano- and polymodal nociceptors signal pain. CTRs are rather interesting (Figure 1). They fire spontaneously at normal corneal temperatures (34–35°C), and under experimental conditions, human CTRs are exquisitely sensitive to transient temperature variations – being able to detect temperature changes of less than half a degree Celsius. The thing is, there’s no evidence that ocular CTRs play any role in environmental temperature assessment in humans or animals. So what are they there for? The answer appears to be detecting the wetness of the eye. When you blink, your ocular surface temperature is ~34°C. That blinking distributes the tear film across the eye, which then evaporates during the interblink period. As evaporation is an endothermic process, this means the temperature of the cornea drops by approximately 0.3°C per second – and it appears that this drop in temperature increases the CTR firing rate and acts as a tonic stimulus for basal tear fluid secretion – likely activating the parasympathetic secretory drive to the lacrimal glands and goblet cells at the superior salivary nucleus. It’s hypothesized that CTR activation acts in the same manner as skin thermoreceptors – they fire at a basal rate, and their input remains subconscious, but when a sufficient number of ocular cold sensory fibers (with CTRs firing at high frequencies) are recruited, people begin to experience conscious sensations of dry eye. It’s therefore easy to see how tear film instability, like in the case of meibomian gland dysfunction, can lead to that unpleasant sensation of dry eye. But that’s not the only issue. Elevated tear osmolarity – another common sequelae of dry eye – also activates CTRs, augmenting their firing rate, and making the sensation of dry eye that bit more unpleasant (4). Worse, as DED progresses, the polymodal nociceptors – which confer the stinging and burning sensations – start to get involved as the cornea starts to become increasingly desiccated and inflammatory processes take hold. Of course, LASIK and PRK both involve transecting corneal nerves, and this might underpin cases of post-photorefractive surgery dry eye: the brain interprets this aberrant activity of these nerves as ocular surface dryness – at least until the corneal nerves regrow after 3–6 months. Imagine if topical NGF could speed that process… And think about this too: people with DED are contraindicated from receiving laser refractive surgery, and are only able to receive it if their DED has been successfully treated. If topical NGF turns out to be an effective dry eye therapy, then this could open the door for far more people to undergo laser vision correction procedures.
NGF and the Posterior Segment
Let’s turn our attention to the back of the eye. As proteins go, NGF is relatively small at 13 kDa – but, given the right formulation, that’s small enough to reach pharmacologically relevant concentrations in the retina after topical administration. Surely, a molecule that promotes the survival and even growth of mature neurons, might be able to play a neuroprotective role in the retina? It appears so. Retinitis pigmentosa Back in 1996, a paper was published by Lambiase et al., which showed that intraocular/retro-ocular mNGF injection partially rescues the photoreceptor loss phenotype of C3H mice – animal models that exhibit photoreceptor degeneration resembling human retinitis pigmentosa (RP) (5). This year, 20 years on from that paper, comes the publication of the first manuscript to describe short-term (10 days) topical mNGF administration in patients with RP (6). Of the eight patients included in the study, three reported “subjective feeling of improved visual performance” that was associated with a temporary enlargement of the visual field in all three patients, and an improved focal ERGs in two of them. Glaucoma In glaucoma, irrespective of intraocular pressure (IOP), the hallmarks of the disease include retinal ganglion cell (RGC) loss and degeneration of the axons which (along with glial cells) form the optic nerve. Ophthalmologists have a variety of approaches for reducing IOP (which remains the only modifiable causative factor in glaucoma) – but can do nothing to address the RGC loss and optic nerve damage that occurs. Can NGF help here? The basic research looks promising. Experimentally-induced glaucoma in in rats (via episcleral vein injections of hypotonic saline) leads to progressive RGC degeneration that’s associated with the downregulation of NGF, TrkA and TrkB – and topical NGF administration has been found to significantly attenuate this deficit (7), promoting RGC survival. As NGF has already been shown to help prevent neuronal degeneration in animal models of other neurodegenerative diseases (8,9), it seems reasonable to assess its use as a neuroprotective strategy for the medical treatment of glaucoma. In 2009, a study was published that described the evaluation of topical mNGF therapy over a period of three months in three patients with advanced glaucoma who were at an “imminent risk of loss of visual function” despite adequate IOP control (10). Electrophysiological and visual acuity tests were performed immediately after the treatment period, and at a three-month follow-up visit. VA was significantly improved after the treatment period (and remained unchanged three months later), and the electrophysiological tests showed that the improvement in post-retinal conduction found at the end of the treatment period was also maintained after three months.NGF’s role in anterior segment pathophysiology
- Mutant mice lacking the high- affinity NGF receptor, TrkA (NTRK1-/-) exhibit reduced corneal sensation, sensitivity and are more prone to corneal lesions
- NGF increases corneal sensitivity in an animal model of refractive surgery
- NGF treatment reverses Capsaicin- induced corneal sensory denervation and healing impairment
- NGF promotes epithelial cell proliferation and differentiation in vitro
- NGF promotes keratocyte differentiation, migration and contraction
- NGF stimulates epithelium healing in vivo
- NGF inhibits apoptosis and upregulates other neurotrophic factors, increasing RGC survival and axonal sprouting
- NGF promotes photoreceptor survival in animal models of retinitis pigmentosa and has shown promise in initial evaluations in patients
- Case reports show promise for NGF treatment in wet AMD
Age-related macular degeneration The preclinical evidence to support the use of NGF in the treatment of AMD is sparse: the first publication of which was a case report back in 2009 (11). A 94-year-old patient with bilateral wet AMD was given topical mNGF over a period of six years in her right eye, but not her left, and visual and electrophysiological assessments were made quarterly. Topical mNGF therapy improved BCVA (both near and distance) and focal ERG amplitude in right eye after only three months of treatment, whereas no improvements in either parameter were observed in the left, untreated, eye. It was actually two years later in 2011, that a paper that investigated the molecular mechanisms involved was published (12). The experiment was as follows: cultured human RPE cells, exposed to hydrogen peroxide undergo apoptosis, something that the addition of NGF protected against. NGF also induced RPE cell migration, which is essential to regenerating damaged parts of the retina. Some insight into the intracellular signaling pathways was also given – the addition of the PI3K/Akt inhibitor LY 294002, or the mTOR inhibitor, rapamycin, blocked the NGF-induced cell survival and migration effects in hydrogen peroxide-treated cells. Given this promising avenue of research, it’s not surprising that NGF isn’t the only neurotrophin under investigation for the treatment of the retinal disease – brain-derived neurotrophic factor (BDNF), fibroblast growth factors (FGFs), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF) and neuropeptide-Y (NPY) are all being investigated – but each of these molecules appear to be expressed in greater amounts when NGF is applied to the retinae of RCS rats (7,13). NGF use is not without adverse effects, though; the most common in the case reports and small trials published so far being a transient, tolerable local corneal irritation. But ultimately, NGF is a molecule that was discovered in the early 1950s, was first successfully used in the clinic in a small number of patients in the mid 1990s, 2000s and 2010s. Now rhNGF can be produced on an industrial scale and is finally undergoing formal clinical trial evaluation for ophthalmic disease. What might NGF in the 2020s bring to the field of ophthalmology? Will it make it to the market? Will it serve only the small number of people with NK? Will those that undergo laser vision correction surgery receive it as part of their postoperative care? Might it delay the progression of glaucoma, or help treat AMD? It’s easy to see how this could be a game-changer, and we have Levi-Montalcini, Cohen and everyone who’s worked on the molecule since to thank for it.
Making rhNGF Mature human NGF isn’t a very big protein – just 118 amino acids – but the fact that it includes three interlaced disulfide bridges means that comes in a complicated package. Often, the production of large quantities of a given protein is relatively simple: clone the gene that encodes the protein into E. Coli, let it express the protein, grow it on an industrial scale, and harvest your protein of interest from the broth. Unfortunately, when human NGF is expressed in E. Coli, it accumulates in the bacteria’s inclusion bodies. The rhNGF can be extracted from the inclusion bodies and folded – but this process is both inefficient and very slow – less than ideal when you require large volumes for pharmaceutical development work, or if wider clinical use is planned. The Italian pharmaceutical company, Dompé, in collaboration with German protein engineering company, Scil Proteins, have developed a considerably more efficient way of producing appropriately folded rhNGF in E. coli cells – resulting in a NGF molecule that’s identical in sequence and structure to the human form.
NGF from Research to Clinic
References
- A Lambiase et al., “Topical treatment with nerve growth factor for corneal neurotrophic ulcers”, N Engl J Med, 338, 1174–1180 (1998). PMID:9554857. S Bonini et al., “Topical treatment with nerve growth factor for neurotrophic keratitis”, Ophthalmology, 107, 1351–1352 (2000). PMID:10889110. A Lambaise et al., “NGF topical application in patients with corneal ulcer does not generate circulating NGF antibodies”, Pharmacol Res, 56, 65–69 (2007). PMID:17512750. C Belmonte, J Gallar, “Cold thermoreceptors, unexpected players in tear production and ocular dryness sensations”, Invest Ophthalmol Vis Sci., 52, 3888–3892 (2011). PMID:21632706. A Lambaise et al., “Nerve growth factor delays retinal degeneration in C3H mice”, Graefes Arch Clin Exp Ophthalmol, 234, Suppl 1, S96-100 (1996). PMID:8871157 B Falsini et al., “NGF eye-drops topical administration in patients with retinitis pigmentosa, a pilot study”, J Transl Med, 14, 8 (2016). PMID: 26748988. V Colafrancesco et al., “Ocular application of nerve growth factor protects degenerating retinal ganglion cells in a rat model of glaucoma”, J Glaucoma, 20, 100–108 (2011). PMID:20436364. CV Borlongan, “Recent preclinical evidence advancing cell therapy for Alzheimer’s disease”, Exper Neurol,237, 142–146 (2012). PMID:22766481. LY Li et al., “Transplantation of NGF-gene-modified bone marrow stromal cells into a rat model of Alzheimer’ disease”, J Mol Neurosci, 34, 157–163 (2008). PMID:18074108. A Lambiase et al, “Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma”, Proc Natl Acad Sci US, 106, 13469–13474 (2009). PMID:19805021. A Lambiase et al, “Nerve growth factor eye drops improve visual acuity and electrofunctional activity in age-related macular degeneration: a case report”, 45, 439–442 (2009). PMID:20061666. GF Cao et al., “Rapamycin sensitive mTOR activation mediates nerve growth factor (NGF) induced cell migration and pro-survival effects against hydrogen peroxide in retinal pigment epithelial cells”, 414, 199–505. PMID:21968016. L Aloe et al., “Nerve growth factor: from the early discoveries to the potential clinical use”, J Transl Med, 10:239 (2012). PMID: 23190582.
