
One major challenge confronting ophthalmology is the restoration of vision in advanced glaucoma and traumatic optic neuropathy (TON) patients. Image formation is dependent on the transmission of visual information to the brain by retinal ganglion cells (RGCs) via their axons. It is these axons which deteriorate or become dysfunctional in glaucoma and TON, resulting in blindness. Although most TON patients do still have an intact and functional imaging system, restoring function in the central nervous system (CNS) following injury or disease depends on the regrowth of axons from surviving neurons. Patients with motor neuron disorders, stroke, viral infection, spinal cord injury, glaucoma, or TON blindness are all impacted by the CNS’ inability to self-repair and face an absence of effective treatments.
Given its accessibility for evaluating RGC damage and recovery, the eye is one of the most desirable models for CNS injury restorative research; numerous researchers have investigated it intensively over the past 30 years (1) . In our own research, we address CNS regeneration in two distinct but synergistic ways. First, we use high throughput multi-omics to identify the proteins, lipids, metabolites, and small molecules involved in CNS injury and regeneration. Second, with the use of computerized natural language processing, we link our results with published research from the last three decades to produce networks that are cohesive and complementary.
Through these two approaches, we’re aiming to create small compounds that can be readily delivered to restore injured nerves. Using the optic nerve of model organisms, we have already observed the regeneration of axons in response to the administration of certain candidates. This exciting preliminary finding is why we have several teams in our Bhattacharya Laboratory currently working on identifying distinct but concerted and convergent pathways and molecules that could promote axon regeneration, ultimately restoring vision.
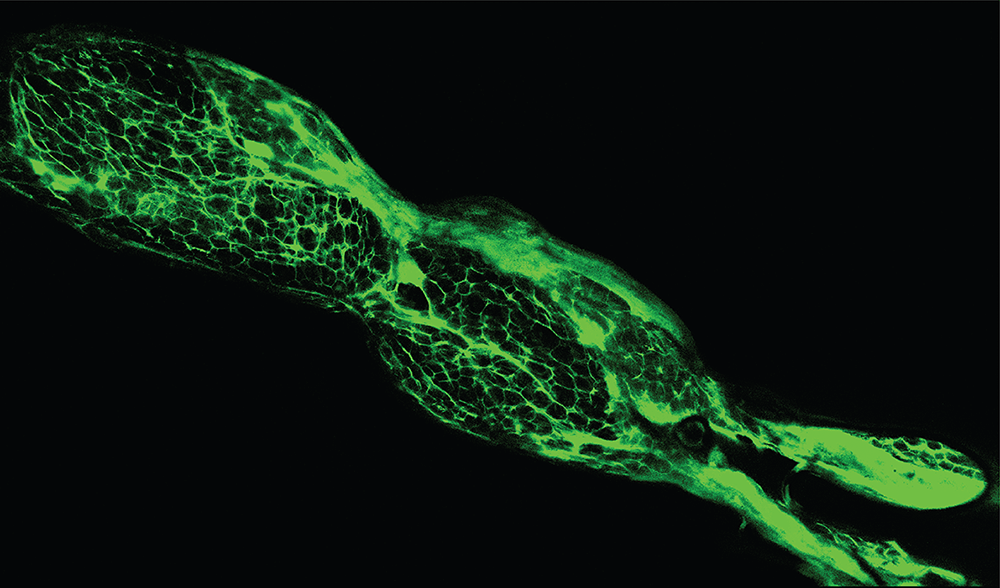
Although our initial results require further validation, they have the potential for a variety of medicinal uses. We also expect that a mixture of molecules will repair axons across a broad age range of patients, which is absolutely crucial in the clinical domain. In particular, we investigated one metabolic pathway, usually investigated in cancer research due to the growth suppression ability of molecules in the pathway and adapted some of its principles to neuron growth. Our objective was to achieve growth through the use of small chemicals without the need for viral vectors and genetic modification and we ultimately discovered molecular (ant)agonists for receptors and downstream factors of growth promoting pathways that produced results that were astonishingly unusual – in a very good way! We saw long-distance growth that encompassed the entire area of the nerve. As shown in Figure 1. the growth is zig-zag, not in a straight line.
Unprecedented growth was induced by one treatment combination, resembling that of mice with defective astrocytes or oligodendrocytes rather than retinal ganglion cells in the optic nerve, resulting in a variety of abnormalities, including improper myelination. The growth promoting effects of these molecules were also confirmed to be transient thanks to endogenous cellular machinery which degrades them, indicating that fine control of the regenerative action of these molecules can be safely exerted over time. Additional research indicates the possibility of an early combinatorial delivery therapy along these lines.
The approach – and initial success – in our laboratory suggest that identification of molecules in the near future can be tested and applied to not just TON patients and ophthalmology as a discipline, but could extend into other areas of the CNS, offering a new approach to spinal cord injuries and other traumatic nerve injuries.
Authors declare no financial conflict of interest.
References
- For some examples of research see: Michele Curcio and Frank Bradke, ‘Axon Regeneration in the Central Nervous System: Facing the Challenges from the Inside,’ Annu Rev Cell Dev Bio, 34, 495 (2018) PMID 30044649; Nancy Newman et al., ‘Understanding the molecular basis and pathogenesis of hereditary optic neuropathies: towards improved diagnosis and management,’ Lancet Neurol 22, 172 (2023) and Mary L Tapia et al., ‘Subtype-specific survival and regeneration of retinal ganglion cells in response to injury,’ Front Cell Dev Biol, (2022).
