
- Age-related eye diseases are multifactorial; some risk factors are modifiable, others are not
- These diseases are lifelong processes, taking decades to develop
- Nutrition plays a role, potentially helping prevent or delay disease progression
- Earlier nutritional supplementation is likely to be more successful than later interventions
We are all aware of the major age-related causes of blindness. Across the globe, cataract, glaucoma, age-related macular degeneration (AMD) and diabetic retinopathy account for three out of four cases of blindness (1). I have two lines of thought that I wish to pursue here. The first is that while they manifest themselves in old age, these diseases are lifelong processes. The second is that they are multi-factorial, caused by a combination of non-modifiable risk factors, such as age and genetic predisposition, and modifiable risk factors, like tobacco smoking, sunlight exposure, cardiovascular factors and nutrition.
Lifelong disease processes
If you were to follow three people from birth; let’s call them Tom, Dick and Harriet (coincidentally, the names of the three tortoises reportedly collected by Charles Darwin during his 1835 visit to the Galápagos Islands), with a single destiny to develop AMD (Tom), glaucoma (Dick), or cataract (Harriet), what would you observe? The very earliest clinical signs of vision-threatening disease would most likely appear in their fifth decade – their forties. Tom might start to exhibit the small yellowish deposits in the retina called drusen; Dick would show elevations in intraocular pressure (IOP), and Harriet would display incipient cataract, a level of lens opacity that she would be unlikely to notice, but would just be detectable on slit lamp/ ophthalmoscopic examination.Return a decade later and the conditions in Tom, Dick and Harriet, now in their fifties, will have advanced noticeably. Numerous large drusen will be detectable under Tom’s retina; Dick’s glaucoma will have progressed to a stage where the retinal nerve fiber thickness has decreased, and Harriet will likely be aware of developing cataracts as her lens opacity reaches an intermediate stage. The seventh decade raises the stakes still higher: Tom’s AMD has become neovascular and atrophic; Dick’s glaucoma has progressed to optic disk cupping, resulting in visual field abnormalities, and the worsening opacity in Harriet’s lenses is increasingly compromising her vision. All three patients are likely to experience major vision loss linked to their diseases in the eighth decade –their seventies. The above assumes that the diseases follow their natural history, without intervention. This is not too far from reality as many people with vision-threatening diseases don’t realize this until significant deterioration in their vision has occurred. In Harriet’s case, cataract surgery and lens replacement drives a great “wow-factor” recovery, but for Tom and Dick the best of today’s interventions simply slow disease progression; typically, if AMD or glaucoma is caught late, what is lost is lost forever. There are two pieces of encouraging news on the prevention front, however. One is that programs promoting regular eye health screenings are effective in saving sight. The other is that improved technology in areas such as adaptive optics imaging and optical coherence tomography could detect the beginnings of these diseases as early as the third decade of life – people in their twenties.
Modifiable risk factors
With the exception of congenital cataract, diseases of blindness are lifelong processes. Many studies have shown that the probability (and rate) of their development is influenced by lifelong exposure to certain risk factors like tobacco smoking and even sunlight. Lifetime sunlight exposure is important to eye health. My research colleagues and I performed a prospective study named Pathologies Oculaires Liées à l’Age (POLA) (2), in which over 2,500 subjects aged 60 years or older recruited from the town of Sète in southern France underwent ophthalmologic examination. Among the parameters measured were lens opacity/ cataract status. We found that increased ambient solar radiation exposure from birth to the age of enrolment into the trial, as assessed by the measurement of solar radiation in the home locations of trial participants throughout their lives, correlated with an increased prevalence of cataracts (Figure 1).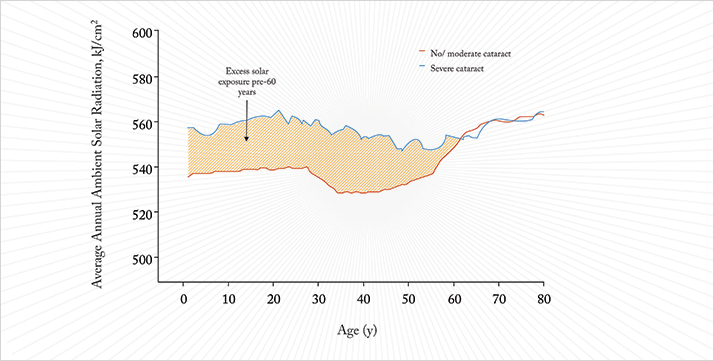
While sunlight exposure is relatively simple to estimate, assessing the impact of diet and nutrition is particularly challenging. Everyone’s tastes are different, and what people eat varies widely from person-to-person and day-to-day. So the only way to get an accurate dietary history would be to have people meticulously note what they eat in a food diary over a period of many decades. This is a huge logistical challenge, and a very costly one so, not surprisingly, we don’t have nutritional data over people’s lifespans. The other option, frequent assessment of nutritional biomarkers (constituents in the blood or urine that can be used to estimate nutrient intake) is also prohibitively expensive. What we do have is longitudinal studies performed in the elderly. My colleagues and I performed one such study, Antioxydants, LIpides Essentiels, Nutrition et Maladies OculaiRes (ALIENOR) in people aged 73 years or more, who live in the Bordeaux region (3). We assessed the participants’ dietary Omega-3 fatty acid intake in 2001–2002 and, six to seven years later, examined their AMD status using retinal photography. Those with a high dietary Omega-3 intake had a 41 percent reduction in risk of late AMD. We also measured participants’ plasma Omega-3 fatty acid levels between 1999–2001 and related it to AMD status using retinal photographs taken between 2006–2008 and 2009–2010. Here, we showed that high plasma Omega-3 fatty acid levels were associated with a 38 percent reduction in the risk of developing late AMD between seven and ten years later (Figure 2).
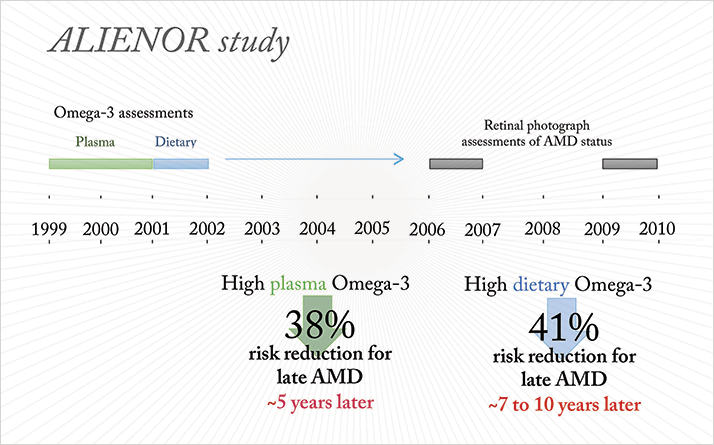
Subsequent studies have confirmed these findings. In subjects with a high genetic risk of developing AMD, pooled analysis of data from the Rotterdam and Blue Mountains studies found that eating fish – the main dietary source of Omega-3 fatty acids – more than once a week, resulted in a 46 percent reduction of developing late AMD over the 15-year period of follow-up data that was available (4).
The impact of dietary supplementation
The Age-Related Eye Disease Studies (AREDS) carried out by the US National Eye Institute (NEI) offer insights into the long-term effects of diet on the development of AMD (5). The original AREDS followed more than 3,600 people who were aged between 55 and 80 years at enrolment and affected either by early AMD or unilateral late AMD. These participants were randomized into four groups that were given placebo, antioxidants (vitamins C and E plus β-carotene), zinc, or antioxidants plus zinc; and they were followed for five years. The primary endpoint was progression to advanced AMD, defined as central geographic atrophy and/ or choroidal neovascularization. At the completion of the study, the antioxidants plus zinc combination had reduced the odds of subjects developing AMD compared with placebo by 28 percent (odds ratio [OR], 0.72; 99% confidence interval [CI], 0.52–0.98). The ORs for zinc alone and antioxidants alone were 0.75 (99% CI, 0.55–1.03) and 0.80 (99% CI, 0.59–1.09), respectively. The positive impact of supplementation may have persisted long after the trial: at 10 years (five years after the initial follow-up period), the risk of developing advanced AMD was 30 percent lower in the group that originally received the antioxidant plus zinc supplements, although subjects from all three groups were free to use any supplement from the end of the trial.In the same AREDS study, a secondary analysis examined the effect of dietary intake of Omega-3 fatty acids, specifically eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). This showed that subjects with the highest (6) baseline EPA/DHA intake had a 35 percent lower risk for developing late AMD over the following 12-year period than subjects with the lowest EPA/DHA intake (Figure 3).
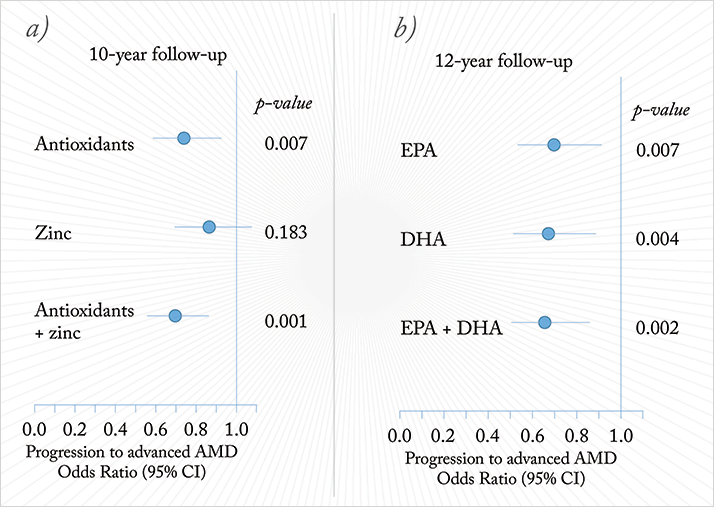
During the course of the AREDS study, β-carotene supplementation was associated with an increased risk of lung cancer (although no such signal was noted in AREDS). This, plus the favorable Omega-3 fatty acid data led to the NEI to perform AREDS2 (7,8). Subjects enrolled were randomly assigned to receive placebo; lutein and zeaxanthin (as alternate carotenoids to β-carotene); DHA plus EPA; or lutein, zeaxanthin, DHA and EPA together. All participants, even those receiving placebo, also received the original AREDS formulation, although a subset of participants also had this formulation altered in a secondary randomization to eliminate β-carotene or reduce the zinc dose. As before, the primary endpoint of the study was the development of advanced AMD, and the initial follow-up period was five years. No significant differences were found between any of the groups, meaning that no supplementation was better than the original AREDS formulation at slowing the progression of AMD. Alterations to the original AREDS formulation did not impact the progression to AMD. There was a significant reduction in lung cancer with the elimination of β-carotene, suggesting that substituting β-carotene with lutein and zeaxanthin was worthwhile. What of the role of Omega-3 fatty acids? AREDS2 found no benefit in preventing AMD, in contrast to many other studies, including sub-analyses of the initial AREDS. However, AREDS2 wasn’t without issues. The study population was atypical in that they were generally highly educated and well-nourished, so perhaps their need for nutritional supplementation was less than other groups. Also, all AREDS2 participants received the original AREDS nutritional supplementation regimen – there wasn’t a no-treatment control group. The Omega-3 fatty acid story hasn’t yet been resolved.
Learning from the young
There have been a number of studies of the very early effects of nutrition on the eye. One, of 379 Australian children aged three to six years, estimated children’s retinal vascular calibers from retinal photographs and measurements of blood pressure and body mass index. The researchers demonstrated that high body mass indices were associated with narrower retinal arterioles and wider retinal venules; similarly elevated blood pressure was associated with narrower arterioles. This work shows the impact of obesity and the effect of body mass index on retinal vessels in children as young as three years old. Perhaps nutritional interventions need to start in the first decade of life? I believe that there is a need for longer-term studies of nutrition in the eye, especially studies evaluating the influence of mid-life nutrition on eye health, as well as the influence of early-life nutrition in children and younger adults. This might help identify the optimal window for nutritional prevention of degenerative eye diseases. Targeting intervention at an elderly population is likely not to be optimal and may explain the lack of effect of nutritional supplementation with Omega-3 fatty acids seen in AREDS2. It is possible that early primary nutritional prevention will be more effective on eye health than secondary prevention (as tested in AREDS and AREDS2) because these are very slow and lifelong processes. Early intervention may be based on nutritional recommendations, but also by acting on the nutritional content of foods that are offered to the public by, for instance, increasing the Omega-3 or antioxidant content of foods. If governments can mandate that all drinking water is fluorinated for the benefit of public dental health, there’s no reason why popular foodstuffs shouldn’t have nutritional supplements added for the benefit of their population’s ocular health.Cécile Delcourt is a Research Fellow at the National Institute for Health and Medical Research (INSERM), Bordeaux University, Bordeaux, France.
References
- S. Resnikoff, D. Pascolini, D. Etya'ale, et al., “Global data on visual impairment in the year 2002”, Bull. World Health Organ., 82, 844–51 (2004). C. Delcourt, I. Carrière, A. Ponton-Sanchez, et al. “Light exposure and the risk of cortical, nuclear, and posterior subcapsular cataracts: the Pathologies Oculaires Liées à l'Age (POLA) study”, Arch. Ophthalmol., 118, 3, 385–92 (2000). C. Delcourt, J.F. Korobelnik, P. Barberger-Gateau, et al., “Nutrition and age-related eye diseases: the Alienor (Antioxydants, Lipides Essentiels, Nutrition et maladies OculaiRes) Study”, J. Nutr. Health Aging, 14, 854–61 (2010). J.J. Wang, G.H. Buitendijk, E. Rochtchina, et al., “Genetic susceptibility, dietary antioxidants, and long-term incidence of age-related macular degeneration in two populations”, Ophthalmology, 121, 667–675 (2014). doi:10.1016/j.ophtha.2013.10.017. Age-Related Eye Disease Study Research Group. “A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation With Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss. AREDS Report No. 8”, Arch. Ophthalmol. 119, 1417–1436 (2001). doi:10.1001/archopht.119.10.1417. J.P. SanGiovanni, E. Agrón, T.E. Clemons, E.Y. Chew, “Omega-3 long-chain polyunsaturated fatty acid intake inversely associated with 12-year progression to advanced age-related macular degeneration”, Arch. Ophthalmol., 127, 110–112 (2009). doi:10.1001/archophthalmol.2008.518. AREDS2 Research Group. “The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1)”, Ophthalmology, 119, 2282–2289 (2012). doi: 10.1016/j.ophtha.2012.05.027. Age-Related Eye Disease Study 2 Research Group, “Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial”, JAMA, 309, 2005–2015 (2013).
