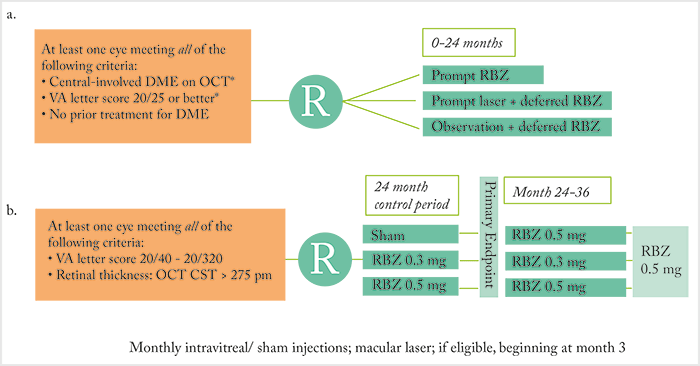
There have been many clinical trials of anti-VEGF agents (and laser therapy) for the treatment of diabetic macular edema (DME) over the years – but still, some questions are unanswered; for example, what is the relationship between anatomic and functional responses to anti-VEGF therapy? Remember Protocol I (Figure 1a [1])? The Phase III multicenter, randomized trial (Figure 1a) showed that ranibizumab (with prompt or deferred focal/grid laser) resulted in superior visual acuity (VA)outcomes compared with laser alone through two years (1). An early post-hoc analysis showed that sustained and early reduction of central retinal thickness (CRT) with ranibizumab therapy was associated with better long-term VA outcomes (2). And yet, asking a similar question, Phase III RIDE/RISE trial (Figure 1b) investigators saw a dissociation between early CRT reductions and visual outcomes (3). As RIDE/RISE also showed that delayed intervention with anti-VEGF therapy limits the scope for future vision improvement, Dugel and colleagues returned to the Protocol I dataset (4) to further characterize the anatomic response of the retina to ranibizumab, as determined by the average extent of edema over the 156-week period post-treatment initiation.
All Protocol I eyes with a baseline and at least one other CRT reading were included in the analysis (n=367). The extent of edema was calculated as the amount by which CRT exceeded 250 μm. Eyes were stratified into quartiles defined by the average extent of edema over the first 52 weeks post-treatment initiation (Figure 2). So what did they find? Edema persisted after treatment initiation – and there was a significant correlation between the average extent of edema in the first and second 52 weeks after treatment initiation (r=0.673; p<0.001), and the average extent of edema that persisted between the first and second 52 weeks post-treatment initiation. In other words, if a patient had a thick macula to begin with, it’s likely that would persist into the second year of treatment. What about VA?
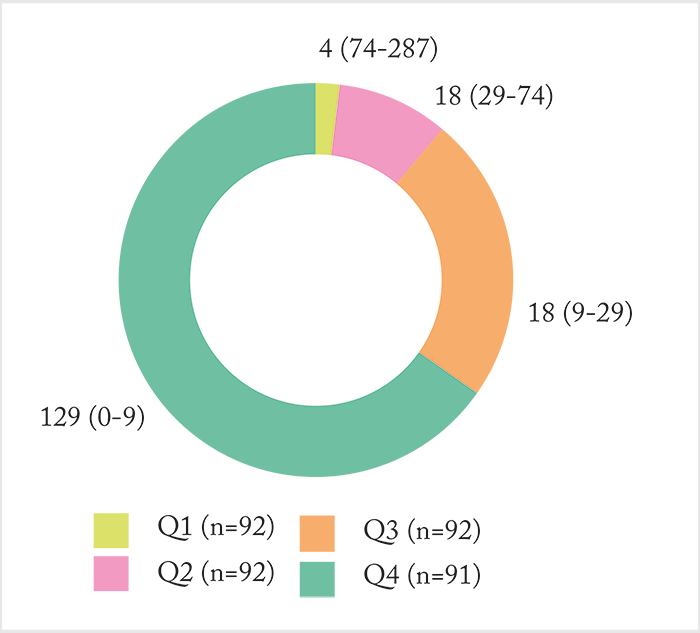
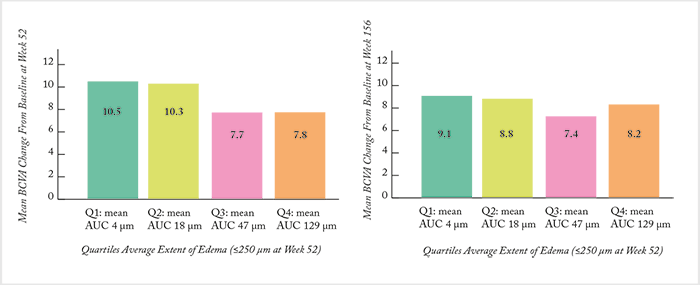
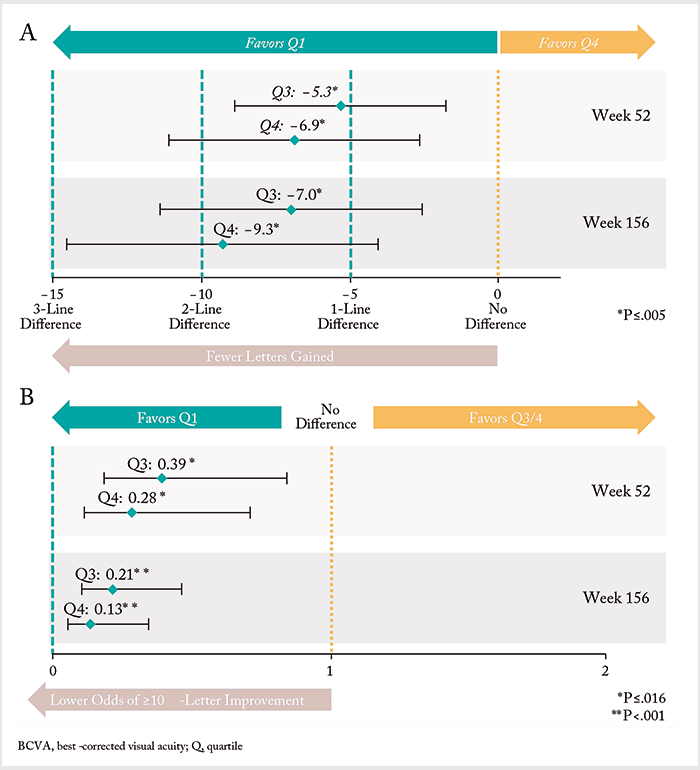
In unadjusted analyses, there was no significant differences in mean BCVA change from baseline at weeks 52 (Figure 3a) and 156 weeks (Figure 3b) across quartiles (p=0.158 and 0.840, respectively). But when the data was split by 52-week quartiles, significant differences in several baseline characteristics (as well as treatment intensity) became apparent. Eyes with the least extent of edema (quartile 1) had better vision and lower CRT at baseline, whereas eyes with the greatest extent of edema (quartile 4) received significantly more ranibizumab injections and laser treatments over the study period. After adjusting for potential confounders (including baseline BCVA and treatment intensity) eyes in quartiles 3 and 4 had a significantly lower estimated mean BCVA change from baseline vs quartile 1 eyes (Figure 4a). Likewise, quartile 3 and 4 eyes also had significantly lower odds of achieving a ≥10-letter improvement in BCVA compared with quartile 1 patients (Figure 4b). The authors’ conclusion? It’s of paramount importance to control for the differences in baseline characteristics between patients with and without an anatomic response. As eyes with the least amount of edema had better baseline VA, the team’s findings may reflect a “ceiling effect,” where eyes with better vision at baseline (on average) have less room to improve. Ultimately, it confirms what RIDE/RISE suggested previously: you should consider additional disease management strategies earlier in eyes that show evidence of persistent edema to maximize potential vision benefit.
References
- MJ Elman et al., “Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema”, Ophthalmology, 117, 1064–1077 (2010). PMID: 20427088. SB Bressler et al., “Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab”, Arch Ophthalmol, 130, 1153–1161 (2012). PMID: 22965591. DM Brown et al., “Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE”, Ophthalmology, 120, 2013–2022 (2013(. PMID: 23706949. PU Dugel et al., “The association between average extent of edema in diabetic macular edema at 52 weeks and long-term vision outcomes: A post hoc analysis of Protocol I”, Presented at: American Society of Retina Specialists 35th Annual Meeting, Boston, August 11–15 (2017).
